Why Test for Mycoplasma in My Cell Culture?
by Fernanda Ruiz, PhD

by Fernanda Ruiz, PhD
Cell culture is a cornerstone technique for biological research laboratories. Cell culture is essential for studying cellular regulatory mechanisms, for stem cell and regenerative medicine studies, and for the generation of biologically active materials including vaccines, enzymes, hormones, and monoclonal antibodies. One very common and often disastrous problem affecting all aspects of cell culture is contamination with other microorganisms. Mycoplasma contamination is of particular concern because it is difficult to detect, often occurs unnoticed in cell cultures, yet it dramatically affects cellular functions.
Mycoplasma are very small, free-living prokaryotes (0.2-0.4 µm) that lack a cell wall, making it impossible to detect them with the naked eye or even through a microscope (Figure 1). In addition, they do not cause cell culture media turbidity, which often accompanies other types of cell culture contamination. Most importantly, Mycoplasma infection generally does not result in observable cell death. Consequently, they can proliferate and go undetected in cell culture dishes for a long period of time, becoming a major obstacle to performing reliable and accurate in vitro experiments.
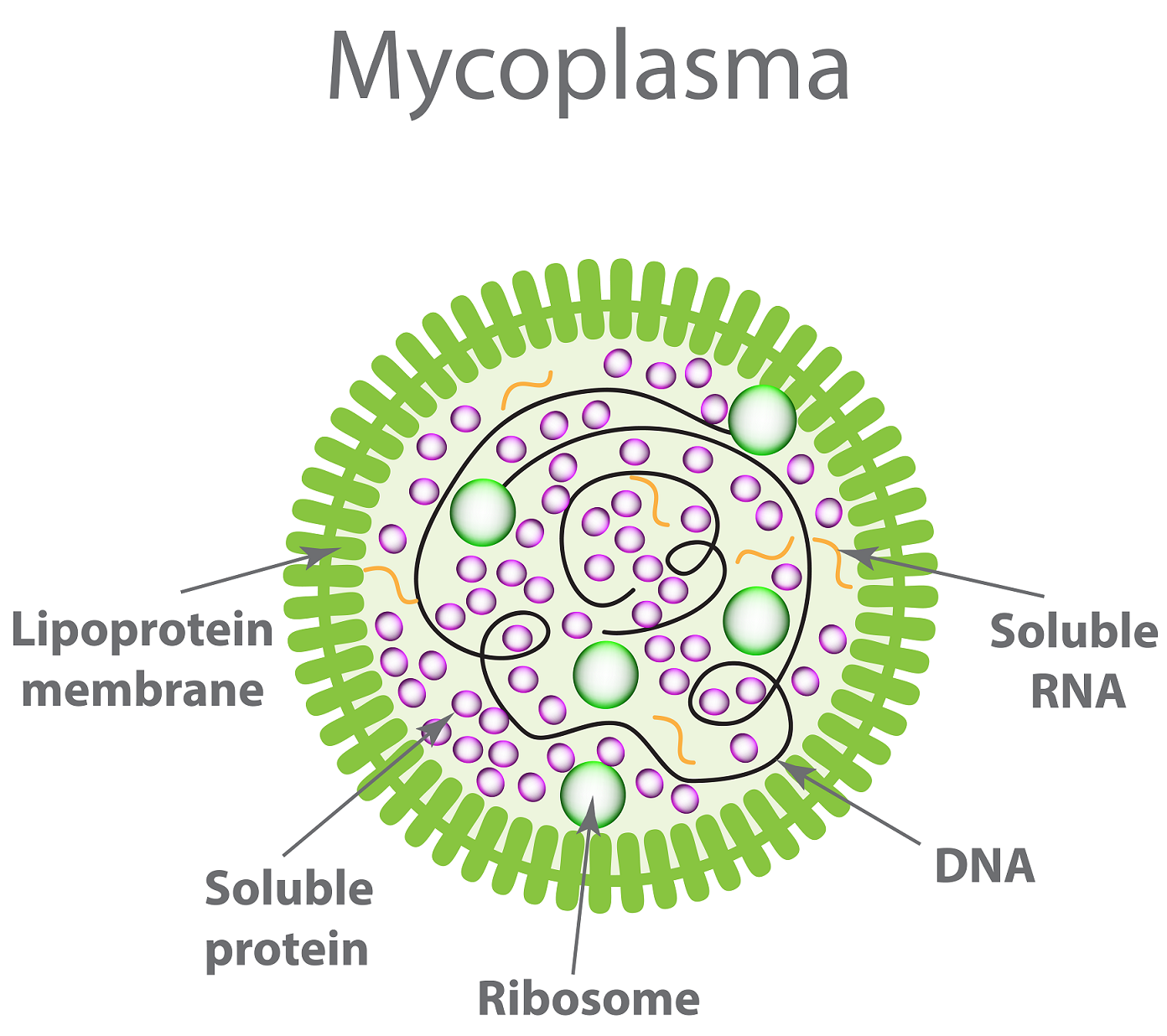
Figure 1. Structure and organelles of Mycoplasma
There are multiple species of Mycoplasma capable of infecting your cell cultures, with the most common being M. orale, M. hyorhinis, M. arginini and Acholeplasma laidlawii. Worldwide, about 15-35% of cell lines are currently contaminated. Today, we know of the existence of >200 Mycoplasma species.
Mycoplasma contamination can affect a cell’s function in many devastating ways (Figure 2). Mycoplasma can alter morphology, metabolism, membrane composition, signal transduction, growth, and viability of cultured cells. In addition, these organisms can affect DNA, RNA and protein synthesis as well as cause chromosomal alterations. Mycoplasma can also reduce the yield of monoclonal antibodies and affect screening of monoclonal antibodies. Thus, mycoplasma’s presence can greatly impede the execution of reliable cell culture assays, impair the production of proteins and prevent generation of reproducible experimental results.
Figure 2. Effects of Mycoplasma on cell cultures.
Finding Mycoplasma in many cell lines throughout the world is not unexpected because it is easily spread and the sources causing the initial infection are numerous. We know that the main source is the introduction of a cell culture already carrying Mycoplasma into the cell culture environment, resulting in Mycoplasma spreading to uncontaminated cell lines (cross-contamination). Another major source is animal sera (fetal bovine serum or newborn bovine serum) and cell culture media. This occurs mainly because these materials are often sterilized by filtration, however, due to their small size, mycoplasmas can pass through some regularly used filters. In addition, the use of nonsterile laboratory materials; incubator and pipettes, for example, can also introduce this organism into your cell culture.
Interestingly, routine handling of cell lines can also result in the introduction or spread of mycoplasmas since humans naturally carry mycoplasmas in their saliva and skin. For example, M. salivarium can be spread through talking or sneezing while handling the cell culture in the incubator. The clothes lab personnel wear can also transport Mycoplasma. Liquid nitrogen is also capable of carrying Mycoplasma and infecting cell lines since cells are routinely stored and preserved in liquid nitrogen. Lastly, exposure to airborne particles can also result in mycoplasma contamination.
Cell culture and the use of cell lines is essential l in biological research and production of biopharmaceutical products. When infection with Mycoplasma occurs, the consequences—infected vaccines, costly drug development interruption, unreliable cell culture assays, irreproducible results, loss of years of work, loss of invaluable cell lines—can be devastating. Furthermore, mycoplasmas are not susceptible to antibiotics commonly used in cell culture. Thus, it is imperative that cell lines are routinely tested on a monthly basis for the presence of Mycoplasma with the most sensitive and reliable method.
Today, cell cultures can be tested for mycoplasma contamination using different methods that have a range of sensitivity and time. Here, we discuss the most common ones (also summarized in Table 1).
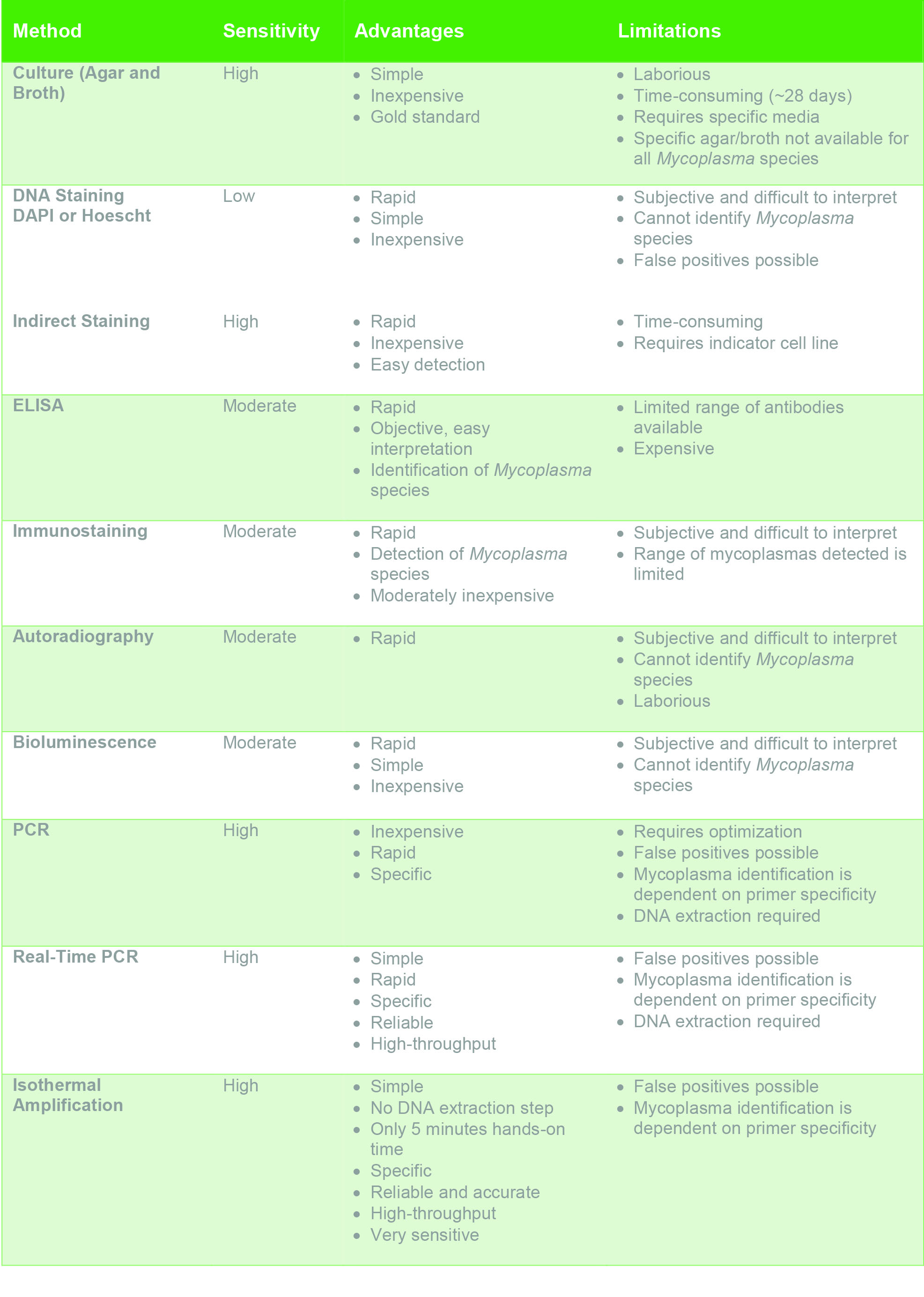
Table 1. Sensitivity, advantages and disadvantages of common mycoplasma detection methods.
This is a common technique and is considered the “Gold standard” of mycoplasma tests. Generally, supernatant from the cell culture to be tested is incubated in mycoplasma culture media then on mycoplasma agar medium for a few weeks. If colonies form after the incubation period, then the sample is considered as positive for Mycoplasma. The advantages include its high sensitivity, simplicity of the test and its cost effectiveness. However, it is laborious, requires specific media preparations and takes a ~28 days to observe colonies, which may be difficult to discern and determine as positive samples. In addition, some Mycoplasma species do not grow well in this agar, which may lead to concluding false negative results.
DNA staining with 4, 6-diamidino-2-phenylindole-dyhydrochloride (DAPI) or Hoechst 33258 is another accepted method routinely used to detect Mycoplasma. First, cells are grown on a cover slip (not to confluency since it is easier to detect mycoplasmas if they are not grown to confluency) then stained with DAPI or Hoechst. Mycoplasma presence is indicated by fluorescence surrounding the cells and fluorescent dots on the cell surface.
This method is easy to perform, fast, and inexpensive. However, its sensitivity is low and determining the presence of Mycoplasma can be subjective. In addition, bacterial contamination and degraded DNA fragments from dead cells in the cell culture can be confused with Mycoplasma. Low levels of contamination may be difficult to detect using fluorescent DNA staining, limiting the sensitivity of this assay.
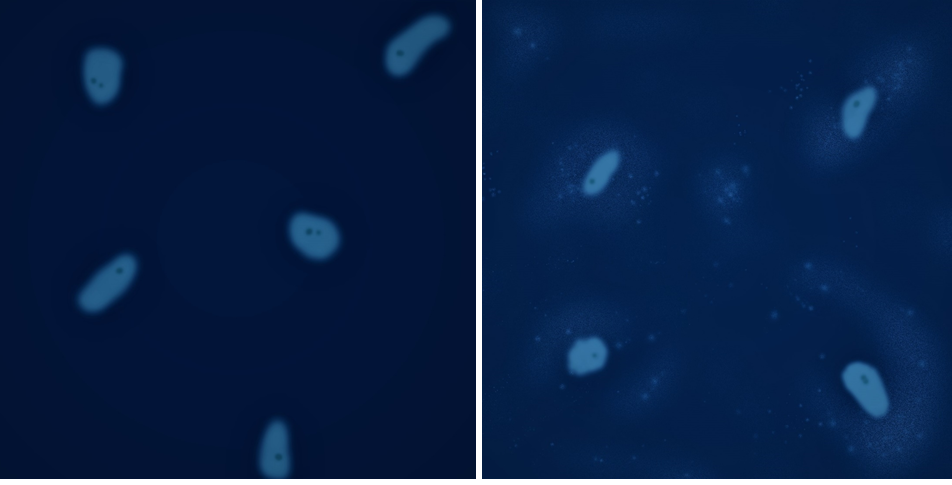 Figure 3. (Illustration) DAPI or Hoechst staining is used to differentiate between uncontaminated (left) and contaminated (right) cell cultures.
Figure 3. (Illustration) DAPI or Hoechst staining is used to differentiate between uncontaminated (left) and contaminated (right) cell cultures.
Indirect DNA staining is a more sensitive alternative to direct DNA fluorescent staining. In this test, indicator cells (NIH-3T3 or Vero B4, for example) are grown with cell media from the cells being tested and then staining is performed on these cultures. In this case, sensitivity increases, however, this assay is time-consuming.
Antibodies raised against Mycoplasma are used in enzyme-linked immunosorbent assay (ELISA). The antibodies can be conjugated with biotin, which binds a streptavidin-alkaline phosphatase complex, leading to hydrolysis of 4-nitrophenyl phosphate. Results are determined using a microplate reader. ELISA can be very helpful in rapidly determining if a cell culture is contaminated and can identify some specific species of mycoplasma. However, the specificity of this method is limited since the range of mycoplasmas that can be identified is limited.
Mycoplasma testing through immunostaining also involves an antibody, in this case, the antibody is raised against an enzyme specifically found on mycoplasmas, such as elongation factor TU (EF-TU). Cells are fixed on a slide and incubated with the biotinylated antibody, which binds specifically to the mycoplasma enzyme if contamination is present. Then, the cells are incubated with streptavidin-FITC. Fluorescence due to mycoplasma presence can then be determined by microscopy.
This method is fast, sensitive and moderately inexpensive. However, just like in DNA staining, interpretation of staining can be subjective, which could result in erroneous conclusions. The ability to detect Mycoplasma is largely dependent on levels of contamination. Immunostaining of cultures with low level of contamination might be challenging to interpret as mycoplasma-contaminated or mycoplasma-free.
Autoradiography, a moderately sensitive technique, requires that cells are grown on cover slips and incubated with an isotope such as [3H] thymidine, for about 3 days. If Mycoplasma is present, nucleoside phosphorylase cleaves thymidine into thymine, which is taken up by Mycoplasma but does not incorporate into mammalian cells. Thus, when Mycoplasma is present, thymidine is converted to thymine, which is taken up by the mycoplasma on the cells’ surface and surroundings, appearing as silver dots in the cytoplasm (extracellular surface since thymine is taken up by mycoplasma) and not in the nucleus.
This method’s advantages include a moderate sensitivity and the ability to see results in a short amount of time. However, this assay required the use of radioactive compounds and interpretation can be subjective and completing the technique is not as simple as other methods.
All living organisms need energy-generating pathways to survive. Mycoplasmas are largely dependent on enzymes such as acetate kinase and carbamate kinase, which convert adenosine diphosphate (ADP) into adenosine triphosphate (ATP). One simple biochemical mycoplasma-detecting test is based on the ADP-ATP reaction mediated by these enzymes, and is coupled with a firefly luciferase bioluminescence reaction. In this method, the presence of Mycoplasma leads to increased ADP-ATP conversion by acetate kinase or carbamate kinase, resulting in a bioluminescent signal that can be detected by a luminometer.
This method is rapid, simple, and inexpensive. Contamination, however, is only reliably detected if the levels of mycoplasma are relatively high and within the limit of detection (LOD) of the test. Another limitation lies in that this test cannot indicate the species of the contaminating mycoplasma.
Polymerase Chain Reaction (PCR) is another common technique used in labs around the world to detect mycoplasma infection in cell cultures. This technique is often preferred to other mycoplasma tests because the procedure is easy, inexpensive, sensitive and specific and reproducible. Primers must be designed so they recognize a range of Mycoplasma species as well as Acholeplasma, but they must specific enough to not amplify sequences from bacteria and other organisms that might have a similar DNA sequence. Also, internal, positive and negative controls must also be included.
PCR does have some drawbacks. PCR often requires optimization and the quality of the sample greatly affects the reliability of the results. Thus, it is advisable to use phenol-chloroform extraction or column extraction to purify the DNA sample. This approach might also lead to false positives since amplification from non-viable mycoplasma or lab contamination with mycoplasma DNA can occur.
Today, Real-Time PCR (qPCR) is a preferred mycoplasma detection test due to it being accurate, sensitive and time-saving. As opposed to routine PCR, qPCR allows observable DNA amplification of mycoplasma DNA in real time as specific primers bind their target sequences in the presence of a fluorescent probe, resulting in amplification of DNA and an increase in fluorescence. Furthermore, qPCR is significantly more specific and sensitive than routine PCR. As in standard PCR, mycoplasma qPCR requires internal, positive and negative controls and can be affected by lab contamination with mycoplasma DNA. Also, this method does require a DNA extraction step.
Isothermal amplification is a detection method that allows for high-throughput, rapid, reliable, and accurate detection of Mycoplasma and Acholeplasma in cell culture without the need for a DNA extraction step. As in qPCR, isothermal amplification requires internal, positive and negative controls. In addition, it requires the same materials as qPCR. Moreover, the sensitivity of this method is much higher, resulting in very accurate results. While this method is more costly, it is also extremely sensitive and can detect very low amounts of mycoplasma leading to very accurate results.

Figure 4. Isothermal amplification procedure for mycoplasma detection.
Whether you suspect cell culture contamination or you are
just performing routine mycoplasma contamination testing, choosing a sensitive
and specific mycoplasma test is essential. Mycoplasma testing should also be
performed when introducing a new cell line, thawing cell lines for experiments,
and when performing experiments for publication. As described previously, the
agar and broth method is considered the “Gold standard.” However, this method
does have great limitations. While also presenting some limitations, recently
developed methods such as Real-Time PCR and isothermal amplification, which are highly sensitive and
specific, do pose as an appealing alternative.
Drexler, H. G., & Uphoff, C. C. (2003). Mycoplasma Contamination Of Cell Cultures. Encyclopedia of Cell Technology. doi: 10.1002/0471250570.spi054.
Hay, R. J., Macy, M. L., & Chen, T. R. (1989). Mycoplasma infection of cultured cells. Nature, 339(6224), 487–488. doi: 10.1038/339487a0.
Kong, F., James, G., Gordon, S., Zelynski, A., & Gilbert, G. L. (2001). Species-Specific PCR for Identification of Common Contaminant Mollicutes in Cell Culture. Applied and Environmental Microbiology, 67(7), 3195–3200. doi: 10.1128/aem.67.7.3195-3200.2001.
EP.2.6.7 (2005) European pharmacopoeia 2.6.7; 2005.
Mariotti, E., Mirabelli, P., Noto, R. D., Fortunato, G., & Salvatore, F. (2008). Rapid detection of mycoplasma in continuous cell lines using a selective biochemical test. Leukemia Research, 32(2), 323–326. doi: 10.1016/j.leukres.2007.04.010.
Mcgarrity, G. J., Vanaman, V., & Sarama, J. (1978). Methods of Prevention, Control and Elimination of Mycoplasma Infection. Mycoplasma Infection of Cell Cultures, 213–241. doi: 10.1007/978-1-4684-9874-5_15.
Studzinski, G. P., Gierthy, J. F., & Cholon, J. J. (1973). An autoradiographic screening test for mycoplasmal contamination of mammalian cell cultures. In Vitro, 8(6), 466–472. doi: 10.1007/bf02615948.
Uphoff, C. C., Gignac, S. M., & Drexler, H. G. (1992). Mycoplasma contamination in human leukemia cell lines. Journal of Immunological Methods, 149(1), 55–62. doi: 10.1016/s0022-1759(12)80048-2.
Uphoff, C. C., & Drexler, H. G. (2014). Detection of Mycoplasma Contamination in Cell Cultures. Current Protocols in Molecular Biology, 106, 28.4.1-28.4.14. doi: 10.1002/0471142727.mb2804s106.
Uphoff, C. C., & Drexler, H. G. (2014). Eradication of Mycoplasma Contaminations from Cell Cultures. Current Protocols in Molecular Biology 106, 28.5.1-28.5.12. doi: 10.1002/0471142727.mb2805s106.
Volokhov, D. V., Graham, L. J., Brorson, K. A., & Chizhikov, V. E. (2011). Mycoplasma testing of cell substrates and biologics: Review of alternative non-microbiological techniques. Molecular and Cellular Probes, 25(2-3), 69–77. doi: 10.1016/j.mcp.2011.01.002.
Young, L., Sung, J., Stacey, G., & Masters, J. R. (2010). Detection of Mycoplasma in cell cultures. Nature Protocols, 5(5), 929–934. doi: 10.1038/nprot.2010.43.
Fernanda Ruiz is a science content writer at Gold Biotechnology. She holds a bachelor's of science in biology from St. Mary's University and a PhD in molecular biology from Baylor College of Medicine.

Covalently conjugating a small molecule to an antibody’s surface is a process called antibody “labeling.” Labeling antibodies with small molecules such as biotin or fluorophores...

DNA gel electrophoresis is one of the most widely used analytical techniques in molecular biology, providing a simple and reliable way to separate DNA fragments...

Do you have a favorite restaurant that you love because you know exactly how great the experience is going to be? There are probably a...

Using the wrong agarose can lead to smearing or poor separation. The good news is that once you know how to evaluate agarose and choose...
