Using Firefly's Light to Find a New Cure
by Tyasning Kroemer, Ph.D.

by Tyasning Kroemer, Ph.D.
Have you ever caught fireflies in the summer and watched as they crawl over your hands? It’s fascinating to watch them flashing their light in the dark.
In the late 1940s, Dr. William McElroy, a scientist at Johns Hopkins University, was curious about this natural phenomenon and paid children to catch fireflies for his research. After accumulating a sizable collection, Dr. McElroy extracted the illuminating compounds essential for the glowing reaction and discovered the science behind the flash.

He saw flashes of greenish-yellow light while conducting his research. The light would flash, and then disappear almost as quickly. To prevent the light from disappearing quickly, he added a compound, Adenosine Triphosphate (ATP). He concluded that ATP acted as an energy source to produce the light in fireflies. After his discovery, Dr. McElroy continued his research on illuminating compounds from fireflies and other organisms.
In the 1980s, Dr. McElroy helped other research teams to clone genes that produce the illuminating enzyme, called luciferase, from beetles. One research team led by Dr. Marlene DeLuca successfully cloned the firefly luciferase gene into several organisms, including Escherichia coli and tobacco plants. Another team cloned the luciferase genes from a Jamaican click beetle into bacteria and made the bacteria glow four different colors (green, yellow-green, yellow, and orange). Since then, scientists around the world have borrowed the light producing chemicals from fireflies to guide their research in a wide range of organisms, from bacteria to parasites.
Fireflies are not the only organism in the world that emits light. Other organisms such as algae, jellyfish, mushrooms, shrimp and glowworms also use give off a beautiful array of multicolored light.
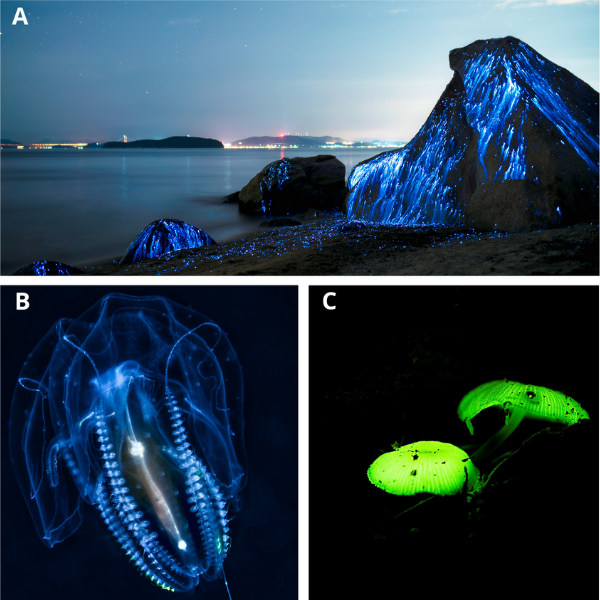
Glowing Organisms: A. Bioluminescent shrimp leave spectacular blue light trails on the beach in Okayama, Japan. B. Bioluminescent jellyfish. C. Bioluminescent mushroom.
The chemical production of light by a living organism is called bioluminescence. Luciferin, meaning light bearer, acts as a substrate (a molecule that an enzyme acts upon) for bioluminescence reactions.
There are two types of bioluminescent reactions produced by organisms. The first type of bioluminescent reaction is the luciferin-luciferase system, in which luciferin is oxidized by the luciferase enzyme in the presence of ATP and oxygen. It leads to the excited state of oxyluciferin and the emission of the light. An example of organisms using the luciferin-luciferase system is the firefly Photinus pyralis.

Luciferin-Luciferase System of the Firefly P. pyralis. Luciferin is oxidized by the luciferase enzyme in the presence of ATP and oxygen. This reaction leads to the excited state of oxyluciferin. As a result, the light is produced.
The second type of bioluminescent reaction doesn’t need a luciferase enzyme, instead the reaction uses a ‘pre-charged’ compound called photoprotein. Photoprotein consists of a substrate, the enzymatic complex, and oxygen. The binding of an agent, typically calcium ions, causes changes of the photoprotein structure. It triggers an oxidative decarboxylation reaction, which leads to the excited state of the product. As a result, this chemical reaction produces the light. The jellyfish Aequorea uses the second type of bioluminescent reaction and requires calcium ions to produce light.

Phototoprotein System of the Jellyfish Aequorea. Three calcium ions bind to the binding sites of photoprotein, changing the photoprotein structure. It leads to the excited state of the product. As a result, this chemical reaction produces light.
Bioluminescence attracts scientists in a wide range of research fields from biotechnology to medical research. Since it is hard to visualize and track small molecules inside the cell (because the appearance of cells and their organelles are typically clear under a microscope), scientists fuse a molecule of interest to a gene that synthesizes a bioluminescent protein, such as luciferase. The scientists can then add luciferin to the cells to make the small molecule visible by producing the glow in the cell.
It’s not surprising that the luciferin-luciferase system has been incorporated into so many different molecular biology methods. Scientists use it to shed light on new discoveries as well as improve many existing research methods. Recent medical research studies have incorporated the luciferin-luciferase system to solve old dilemmas, such as antibiotic resistance or anti-parasite drug resistance.
Antibiotic resistance develops when populations of microbes acquire genes that protect against antibiotics that were originally able to kill them. According to Centers for Disease Control and Prevention (CDC), at least 2.8 million people are infected with antibiotic-resistant bacteria, and more than 35,000 people die each year in the U.S. because of antibiotic resistance (CDC, 2019). The major causes of the antibiotic resistance are the overuse or misuse of antibiotics and the ability of bacteria to transfer the antibiotic resistance genes to other bacteria. To complicate things further, some bacteria develop resistance to more than one class of antibiotic.
One approach to overcome the resistance problem is to find a new antibiotic or a more effective concentration of the antibiotic. The most important testing for new antibiotic discovery is an antibiotic susceptibility testing, performed by exposing members of bacteria families to a particular antibiotic. However, the antibiotic susceptibility test must be accurate since any false results can prove fatal for a patient.
There are three commonly used tests for antibiotic susceptibility testing:

Disc Diffusion Method (Kirby-Bauer Test). Bacteria are grown in growth media agar plates. Discs containing antibiotics are placed on the surface of the agar. Zone of inhibition, a clear area around the disc, is measured.

Etest. Bacteria are grown in growth media agar plates. A test strip containing a gradient antibiotic concentration is placed onto the surface of the agar. A point at which the zone of inhibition intersects with the test strip is called minimum inhibitory concentration (MIC).
A rapid susceptibility test could save a lot of time and help find a new cure before bacteria start developing resistance. Heller and Spence (2019) reported a rapid susceptibility test based on bioluminescence that utilizes an optical density measurement at 600 nm and the release of ATP by bacteria.
When the bacteria grow, the extracellular ATP increases until the late logarithmic to early stationary phase. During the stationary phase, the extracellular ATP decreases.
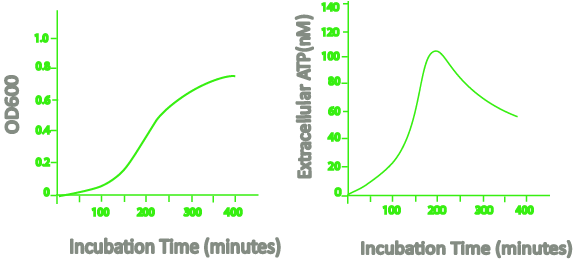
Diagram of OD600 and extracellular ATP level during bacteria incubation time (Heller and Spence, 2019). The extracellular ATP level of Escherichia coli bacteria shows the increasing followed by decreasing pattern.
This increasing followed by decreasing pattern of extracellular ATP only occurs in living bacteria. The concentration of ATP remains constant during the stationary phase when the bacteria are dead.
In the reported bioluminescence method, the ATP concentration was measured based on the luciferin-luciferase system with luciferin as a substrate. When the ATP produced by bacteria increased, the amount of light measured also increased. The advantage of this bioluminescence method is it takes a very short time to get a result (less than 1 hour after the addition of the antibiotic) compared to the standard methods of resistance detection.
World Health Organization reported in 2018 there were an estimated 228 million cases of malaria worldwide and the estimated number of malaria deaths was at 405,000. It doesn’t help when the development of resistance against anti-malarial drugs and insecticides keep increasing. Discovering a new anti-malarial drug or a malaria vaccine is one of many possible solutions to help the ongoing effort to treat this terrible disease. A biological assay as an initial or secondary screen is required to test a new drug or vaccine in animal models, such as mice. To perform this bioassay, scientists must develop a rapid (less than one hour) and accurate way to quantify the presence of parasites in the animal blood (parasitaemia).
Three commonly used tests to detect parasitaemia in mice are:
In 2019, Caridha et al. developed an improved method to quantify parasitaemia in mice, measuring the bioluminescence signal in treated mice. In their research, mice were infected with transgenic malaria parasites producing luciferase. The mice were then injected with luciferin at 10 minutes prior to imaging so that the luciferase-luciferin chemical reaction inside the mice produced a measureable bioluminescence signal. The signal increased in mice for up to 7 days post-infection, meanwhile it took around 6 days post-infection for some mice to show severe malaria symptoms.
The use of this bioluminescence method can help scientists to follow the progression of parasitaemia to detect sick mice that have yet to show any obvious malaria symptoms. This method is relatively simple and faster compared to other methods, although it requires special equipment to detect the bioluminescence signal.
Dr. William McElroy might have started his research on light production of fireflies out of curiosity, and his discovery might have only been about a basic chemical reaction. However, there is no doubt that if he hadn’t taken an interest in investigating this phenomenon, the luciferin-luciferase system wouldn’t have become the helpful research tool for scientists and scientific research that it is today.
Caridha, D., Hickman, M., Xie, L., Ngundam, F., Milner, E., Schenk, A., et al. (2019). Updating the modified Thompson test by using whole-body bioluminescence imaging to replace traditional efficacy testing in experimental models of murine malaria. Malaria Journal, 18(1), 38. doi:10.1186/s12936-019-2661-x.
CDC. (2018, September 10). About Antimicrobial Resistance. Retrieved from Centers for Disease Control and Prevention website: https://www.cdc.gov/drugresistance/about.html
Citron, D. M., Ostovari, M. I., Karlsson, A., & Goldstein, E. J. (1991). Evaluation of the E test for susceptibility testing of anaerobic bacteria. Journal of clinical microbiology, 29(10), 2197.
Deluca, M., & McElroy, W. D. (1978). [1] Purification and properties of firefly luciferase. In Methods in Enzymology (Vol. 57, pp. 3-15): Academic Press.
de Wet, J. R., Wood, K. V., Helinski, D. R., & DeLuca, M. (1985). Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proceedings of the National Academy of Sciences, 82(23), 7870. doi:10.1073/pnas.82.23.7870.
Dybas, C. L. (2019). Illuminating New Biomedical Discoveries: Bioluminescent, biofluorescent species glow with promise. BioScience, 69(7), 487-495. doi:10.1093/biosci/biz052.
Hänscheid, T., & Grobusch, M. P. (2002). How useful is PCR in the diagnosis of malaria? Trends in Parasitology, 18(9), 395-398. doi:https://doi.org/10.1016/S1471-4922(02)02348-6.
Hastings, J. W. (2004). William David McElroy. Biographical Memoirs. vol. 85. National Academy of Sciences. pp. 164–183.
Heller, A. A., & Spence, D. M. (2019). A rapid method for post-antibiotic bacterial susceptibility testing. PloS one, 14(1), e0210534-e0210534. doi:10.1371/journal.pone.0210534.
Jenkins, R., & Maddocks, S. (2019). Chapter Four - Antimicrobial testing. In R. Jenkins & S. Maddocks (Eds.), Bacteriology Methods for the Study of Infectious Diseases (pp. 73-97): Academic Press.
Jorgensen, J. H. (1993). Selection criteria for an antimicrobial susceptibility testing system. Journal of clinical microbiology, 31(11), 2841-2844.
Khan, A. Z., Siddiqui, F. M., & Park, S. (2019). Current and Emerging Methods of Antibiotic Susceptibility Testing. Diagnostics, 9(2). doi:10.3390/diagnostics9020049.
McElroy, W. D. (1947). The Energy Source for Bioluminescence in an Isolated System. Proceedings of the National Academy of Sciences of the United States of America, 33(11), 342-345. doi:10.1073/pnas.33.11.342.
Mempin, R., Tran, H., Chen, C., Gong, H., Kim Ho, K., & Lu, S. (2013). Release of extracellular ATP by bacteria during growth. BMC microbiology, 13, 301-301. doi:10.1186/1471-2180-13-301.
Miller, J. L., Murray, S., Vaughan, A. M., Harupa, A., Sack, B., Baldwin, M., et al. (2013). Quantitative bioluminescent imaging of pre-erythrocytic malaria parasite infection using luciferase-expressing Plasmodium yoelii. PloS one, 8(4), e60820-e60820. doi:10.1371/journal.pone.0060820.
Moody, A. (2002). Rapid Diagnostic Tests for Malaria Parasites. Clinical Microbiology Reviews, 15(1), 66. doi:10.1128/CMR.15.1.66-78.2002.
Ohmiya, Y., & Hirano, T. (1996). Shining the light: the mechanism of the bioluminescence reaction of calcium-binding photoproteins. Chemistry & Biology, 3(5), 337-347. doi:https://doi.org/10.1016/S1074-5521(96)90116-7.
Ow, D. W., De Wet, J. R., Helinski, D. R., Howell, S. H., Wood, K. V., & Deluca, M. (1986). Transient and Stable Expression of the Firefly Luciferase Gene in Plant Cells and Transgenic Plants. Science, 234(4778), 856. doi:10.1126/science.234.4778.856.
Ozawa, T., Yoshimura, H., & Kim, S. B. (2013). Advances in Fluorescence and Bioluminescence Imaging. Analytical Chemistry, 85(2), 590-609. doi:10.1021/ac3031724.
Reller, L. B., Weinstein, M., Jorgensen, J. H., & Ferraro, M. J. (2009). Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clinical Infectious Diseases, 49(11), 1749-1755. doi:10.1086/647952.
Sandle, T. (2016). 14 - Antibiotics and preservatives. In T. Sandle (Ed.), Pharmaceutical Microbiology (pp. 171-183). Oxford: Woodhead Publishing.
Seliger, H. H., & McElroy, W. D. (1964). The Colors of Firefly Bioluminescence: Enzyme Configuration and Species Specificity Proceedings of the National Academy of Sciences of the United States of America, 52(1), 75-81. doi:10.1073/pnas.52.1.75.
Shimomura, O., Johnson, F. H., & Saiga, Y. (1962). Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. Journal of Cellular and Comparative Physiology, 59(3), 223-239. doi:10.1002/jcp.1030590302.
Shimomura, O., & Johnson, F. H. (1970). Calcium Binding, Quantum Yield, and Emitting Molecule in Aequorin Bioluminescence. Nature, 227(5265), 1356-1357. doi:10.1038/2271356a0.
Sierzputowski, K. (2016, August 18). Blue Rivers of Bioluminescent Shrimp Trickle Down Oceanside Rocks in Okayama, Japan. Retrieved from https://www.thisiscolossal.com/2016/08/glowy-shrimp-japan.
Malaria. (2019). World Health Organization. https://doi.org//entity/malaria/en/index.html.
Wood, K. V., Lam, Y. A., Seliger, H. H., & McElroy, W. D. (1989). Complementary DNA coding click beetle luciferases can elicit bioluminescence of different colors. Science, 244(4905), 700. doi:10.1126/science.2655091.

Making IPTG stock solution involves weighing out IPTG powder, pouring it into a conical tube or cylinder, and adding deionized water to the desired volume....

IPTG and auto-induction are two ways to induce protein expression in bacteria. They work similarly, but have different trade-offs in terms of convenience. While IPTG...

The final concentration of IPTG used for induction varies from 0.1 to 1.0 mM, with 0.5 or 1.0 mM most frequently used. For proteins with...

A His-tag is a stretch of 6-10 histidine amino acids in a row that is used for affinity purification, protein detection, and biochemical assays. His-tags...
