A Quick and Helpful Introduction to the ATP Bioluminescence Assay
by Katharine Martin

by Katharine Martin
Cell viability assays are used by many researchers to evaluate cell health. This assay category is useful for experimental optimization, understanding cell metabolism, studying the effects of a certain treatment, etc. In this article, we will specifically focus on the ATP bioluminescence assay, which uses the relationship between ATP, luciferin and luciferase to help determine the number of live cells.
What is the ATP bioluminescence assay?
How does the ATP bioluminescence assay work?
What applications use the ATP bioluminescence assay?
What substrates are used for the ATP bioluminescence assay?
ATP bioluminescence substrate challenges
The ATP bioluminescence assay is based on the presence of ATP in metabolically active (living) cells, as intracellular ATP rapidly diminishes after cell death. By measuring ATP-dependent bioluminescence, researchers can estimate the number of viable cells within a sample.
In the case of a living cell, which has ATP, the luciferin-luciferase reaction would produce a bioluminescent flash. In the case of a dead cell, which does not have ATP, no bioluminescence reaction would occur.
Under controlled conditions, the bioluminescence signal correlates linearly with ATP concentration, which in turn reflects the number of viable cells in the sample (Brovko, 2010).
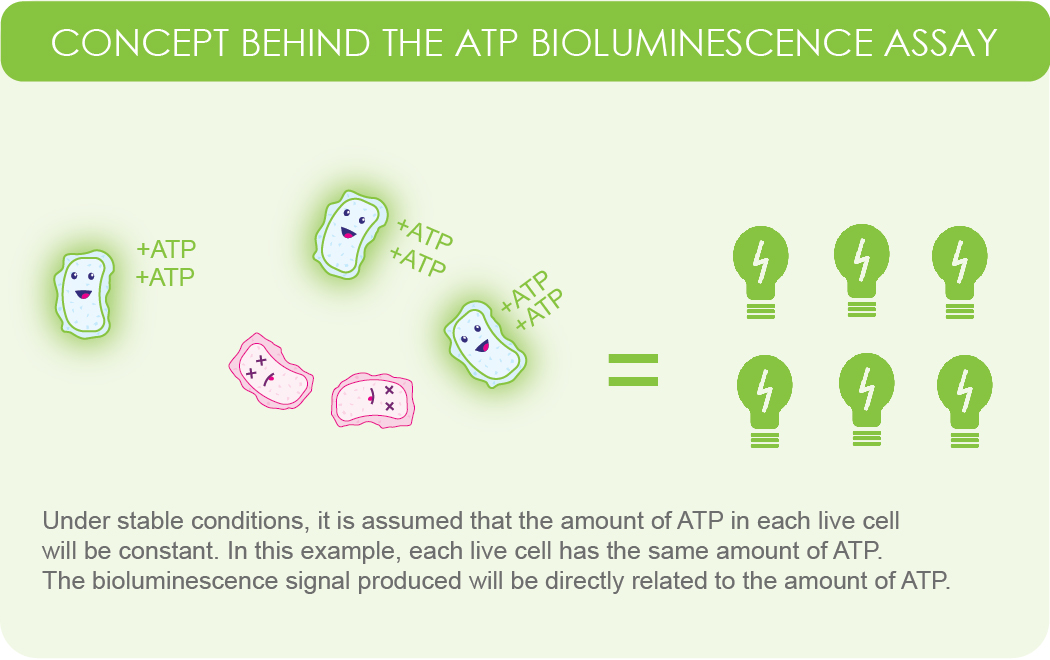
Figure 1. Simplified illustration showing the assumption that
live cells within a sample will produce a constant amount of ATP. Because the reaction requires ATP, the signal intensity will be proportional to the amount of ATP in the sample.
The ATP bioluminescence assay works by exposing a sample of cells to the enzyme luciferase and the substrate luciferin. Because the luciferase reaction relies on ATP, only living cells in the sample would produce a bioluminescent signal.
Alternatively, in the case of dead cells, cell metabolism is disrupted. However, ATPases (enzymes that degrade ATP) still function. This leads to a quick drop in ATP in dead cells within the sample.
But to really understand how the ATP bioluminescence assay works, we need to understand the luciferase reaction.
There are two steps in the luciferase reaction. The first step is the adenylation of the substate luciferin which uses ATP. The second step is the oxidative decarboxylation of the luciferyl adenylate to form oxyluciferin. The oxyluciferin formed is at an excited state, and light is produced when it moves back to the ground state (Belanger & University of Rochester Introductory Biochemistry, 2018).

Figure 2. Shows the basic firefly luciferase reaction involving ATP and D-luciferin. Once the excited oxyluciferin reaches ground state, light is produced.
What this shows us is that ATP is a fundamental compound within the luciferase reaction. Knowing this, and the fact that ATP exists within living cells, researchers are able to utilize this reaction and get visual data about the number of living and dead cells within a sample.
The ATP bioluminescence assay is used for many studies such as (Riss et al., 2016), (Brovko, 2010):
The ATP bioluminescence assay relies on the luciferase reaction. Therefore, the substrates used will be firefly luciferin.
Keep in mind, there are other bioluminescent cell viability assays that use different substrates and compounds.
Renilla and Gaussia based cell viability assays use the substrate coelenterazine. One of the advantages to this method is that Gaussia is secreted from the cell, making it easier for in vivo detection and high-throughput screening. However, a limitation of Renilla and Gaussia-based assays is that their emission (~480 nm) is strongly absorbed and scattered in mammalian tissues, reducing sensitivity for in vivo imaging of deep tissues (Brovko, 2010).
While luciferin does easily permeate the eukaryotic cell membrane, challenges arise when dealing with the cell wall of a bacterial cell. This is because in solution luciferin is negatively charged.
One way around this is to lower the pH to a slightly acidic level. Doing so allows protonation of the carboxylic group of luciferin. Be careful not to lower the pH below 5.0, which will inactivate luciferase.
Another method of luciferin introduction into bacterial cells is by using polymyxin B. Polymyxin B creates pores in E. coli cells that allow luciferin to penetrate the cell. Again, there is a note of caution because you do not want to use a lethal concentration.
Finally, caged luciferins are another way to introduce luciferin into a bacterial cell. These modified substrates are neutral and are therefore more permeable. Upon entrance into the cell they can be converted into the active form either by an enzymatic process or by photoactivation (Brovko, 2010).
AIB Staff. (2013, October 11). A Q&A on ATP Bioluminescence assay. Retrieved March 22, 2021, from https://www.qualityassurancemag.com/article/aib1013-atp-bioluminescence-assay/
Belanger, S., & University of Rochester Introductory Biochemistry (Producers). (2018, May 15). Luciferase [Video file]. Retrieved March 22, 2021, from https://www.youtube.com/watch?v=odN92KbH8Bo
Brovko, L. (2010). Bioluminescence and fluorescence for in vivo imaging: In vivo optical imaging (Vol. TT91). Bellingham, WA, Washington: SPIE Press.
Riss, T., Moravec, R., Niles, A., Duellman, S., Benink, H., Worzella, T., & Minor, L. (2016). Cell Viability Assays. The Assay Guidance Manual.

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...

The GoldBio Floating Tube Rack is one of our more clever giveaways because of the unique purpose it serves. And, with it also being one...

The characteristic blue color of nickel agarose beads comes from the 2+ oxidation state of the nickel ions. Color is also a useful indicator for...
