GelRed vs. Ethidium Bromide: A Comprehensive Look
by GoldBio Marketing Team

by GoldBio Marketing Team
Your electrophoresis routine has become second nature. You mix the buffer, melt the agarose, and reach for that familiar bottle of ethidium bromide (EtBr). You know there are some concerns about it, and you take the normal precautions. But after researching the safer alternative GelRedTM, you now have some questions.
For instance, you're probably wondering what is the difference between GelRedTM and Ethidium Bromide?
GelRedTM is a safer, more sensitive alternative to ethidium bromide that uses two ethidium subunits connected by a spacer to prevent cell membrane penetration while maintaining nearly identical fluorescent properties and offering enhanced sensitivity for DNA detection.
This comparison matters because DNA staining is fundamental to molecular biology workflows, and the choice between these dyes impacts both laboratory safety protocols and experimental results. Understanding the key differences helps researchers make informed decisions that balance safety, performance, and cost considerations.
In this article, we will examine the structural differences between these dyes, analyze their safety profiles, compare their performance characteristics, and provide guidance on when to choose each option for your specific applications.
What is Ethidium Bromide and How Does it Work?
What is GelRedTM and How Does it Work?
How Are GelRedTM and Ethidium Bromide Structurally Different?
How Does Structural Difference Make GelRedTM Safer?
How Do GelRedTM and Ethidium Bromide Compare in Performance?
What Are the Practical Considerations for Each Dye?
When Should You Choose GelRedTM vs. Ethidium Bromide?
Ethidium bromide (EtBr) has been the gold standard for DNA. EtBr's phenanthridinium ring system and amino groups both cooperate to intercalate between DNA base pairs (Figure 1).
EtBr works by intercalating between the base pairs of DNA. Binding to DNA enhances fluorescence and is visualized when excited with UV light (Olmsted & Kearns, 1977).
Ethidium bromide has remained popular in molecular biology laboratories due to its high sensitivity, low cost, and reliable performance across various gel types and experimental conditions.
GelRedTM is a next-generation nucleic acid stain developed as a safer alternative to ethidium bromide while maintaining nearly identical optical properties.
Structurally, GelRedTM consists of two ethidium subunits connected by a linear oxygenated spacer chain.
The binding mechanism involves intercalation similar to ethidium bromide, but the dimeric structure with two DNA-binding units connected by the spacer chain provides enhanced binding affinity (Figure 1).
Research confirmed that GelRedTM binds DNA mainly through intercalation (Crisafuli et al., 2015).
GelRedTM is compatible with both standard UV transilluminators and blue light imaging systems, requiring little to no equipment modifications for laboratories switching from ethidium bromide.
The dye works effectively with both agarose and polyacrylamide gels and is suitable for visualizing double-stranded DNA, single-stranded DNA, and RNA, though sensitivity for single-stranded DNA and RNA can be lower than for double-stranded DNA (this is true for both dyes).
The fundamental structural difference lies in GelRedTM's dimeric design compared to ethidium bromide's monomeric structure.
Ethidium bromide contains a single phenanthridinium ring system, while GelRedTM consists of two ethidium-like units connected by a polyethylene glycol (PEG) spacer chain, creating a much larger molecular structure that prevents cellular uptake.
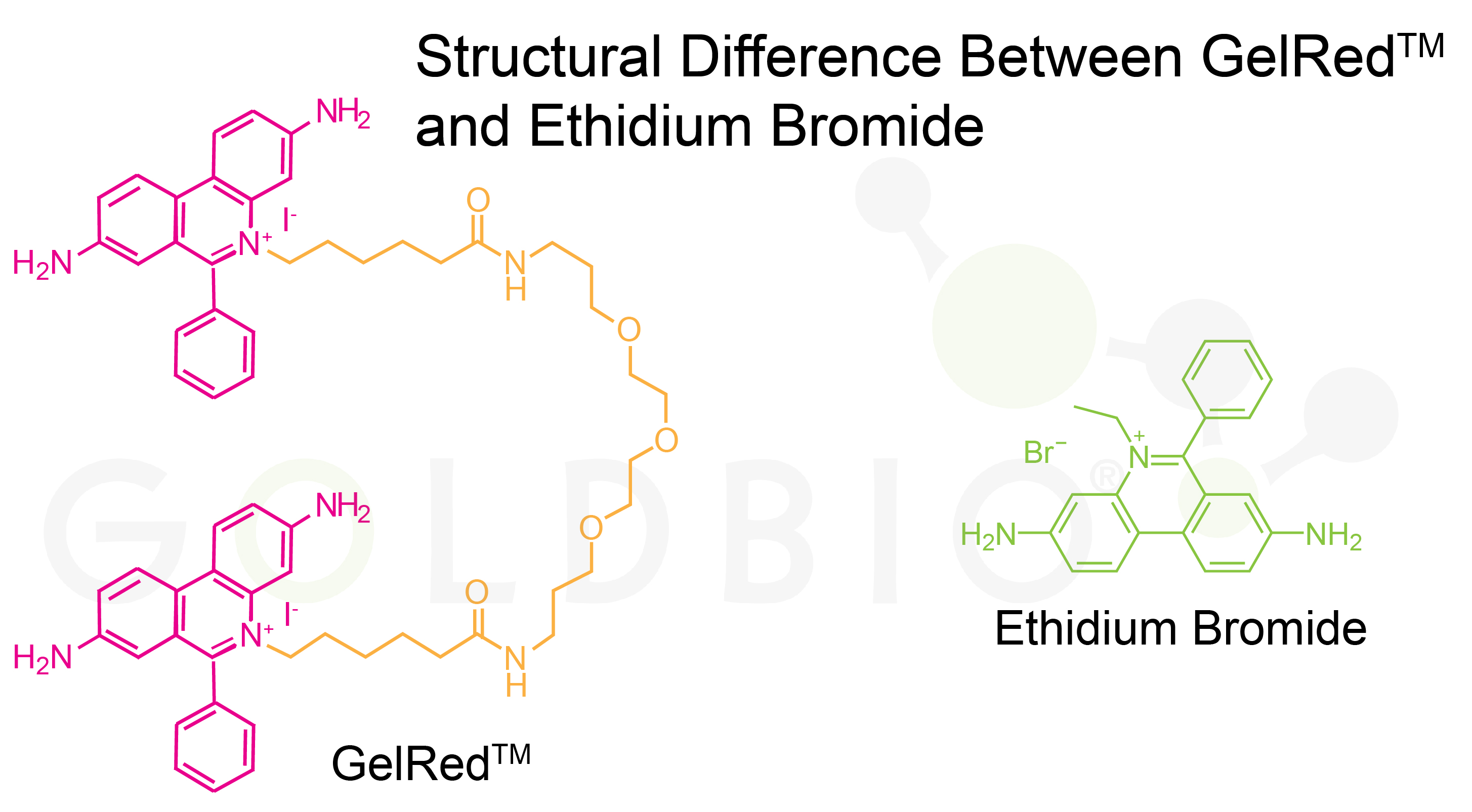
Figure 1. GelRedTM and Ethidium Bromide
molecules, showing structural differences.
The PEG spacer in GelRedTM increases the molecule's hydrophilicity and size. These modifications were designed to prevent the molecule from crossing hydrophobic cell membranes while preserving the DNA-binding and fluorescent properties of the ethidium units.
The larger molecular size and reduced membrane permeability of GelRedTM directly translates to its improved safety profile compared to ethidium bromide.
GelRedTM's inability to penetrate cell membranes due to its large molecular weight is the primary safety advantage that prevents DNA binding in living cells, while ethidium bromide's smaller size allows cellular uptake and DNA intercalation in vivo.
The safety advantage stems from several structural factors:
Size Exclusion: GelRedTM is a bulkier molecule that cannot pass through intact cell membranes, preventing access to cellular DNA. Ethidium bromide's compact structure allows it to enter cells and potentially cause mutations by intercalating with chromosomal DNA.
Membrane Impermeability: The hydrophilic spacer chain and overall molecular architecture of GelRedTM creates a molecule that remains external to cells. Testing by independent laboratories confirmed that GelRedTM at working concentrations is non-toxic, non-mutagenic, and non-hazardous to aquatic life (Biotium, 2013).
This structural design represents a clever approach to maintaining fluorescent properties while eliminating the primary safety concern associated with ethidium bromide use.
Something we want to note when it comes to safety is ethidium bromide’s level of hazard has been long debated. But GelRedTM can offer more explicit safety features and peace of mind because its inability to penetrate cellular DNA makes it even less toxic.
Performance characteristics reveal both similarities and important differences between these DNA staining options.
GelRedTM has been reported to offer equal or greater sensitivity than ethidium bromide, often allowing detection of as little as 0.25 ng DNA per band under optimal conditions.
Nucleic Acid Compatibility: GelRedTM works with double-stranded DNA, single-stranded DNA, and RNA, with twice the sensitivity for double-stranded DNA compared to single-stranded nucleic acids. Ethidium bromide shows similar compatibility but with generally lower overall sensitivity.
Background Fluorescence: Both ethidium bromide and GelRedTM exhibit similarly low background fluorescence in properly prepared gels.
The enhanced sensitivity of GelRedTM can be particularly advantageous when detecting low-abundance DNA fragments.
Real-world implementation involves several practical factors beyond basic performance metrics.
Cost considerations favor ethidium bromide initially, but GelRedTM's enhanced sensitivity and simplified disposal may offset higher upfront costs.
Keep in mind, however, that GelRedTM is not federally regulated as hazardous waste in the US (at the time of writing this), but you always want to follow your local guidelines.
Protocol Differences: Both dyes can be used in identical protocols; however, some optimization may be needed when switching to GelRedTM. This article goes into more detail about ways to get better results when using GelRedTM.
Overall, general protocol compatibility simplifies laboratory transitions between dyes. Both dyes are pH-sensitive and perform optimally around pH 8-9, which matches standard electrophoresis buffer conditions.
Storage Considerations: Both dyes are typically supplied as concentrated stock solutions that remain stable for extended periods when stored properly at room temperature and protected from light.
Selecting the appropriate DNA stain depends on multiple factors specific to your laboratory environment and experimental needs.
Choose GelRedTM when safety is prioritized, institutional policies favor non-toxic alternatives, high sensitivity is required, or simplified waste disposal offers significant advantages. Ethidium bromide still remains suitable for cost-sensitive applications with established safety protocols.
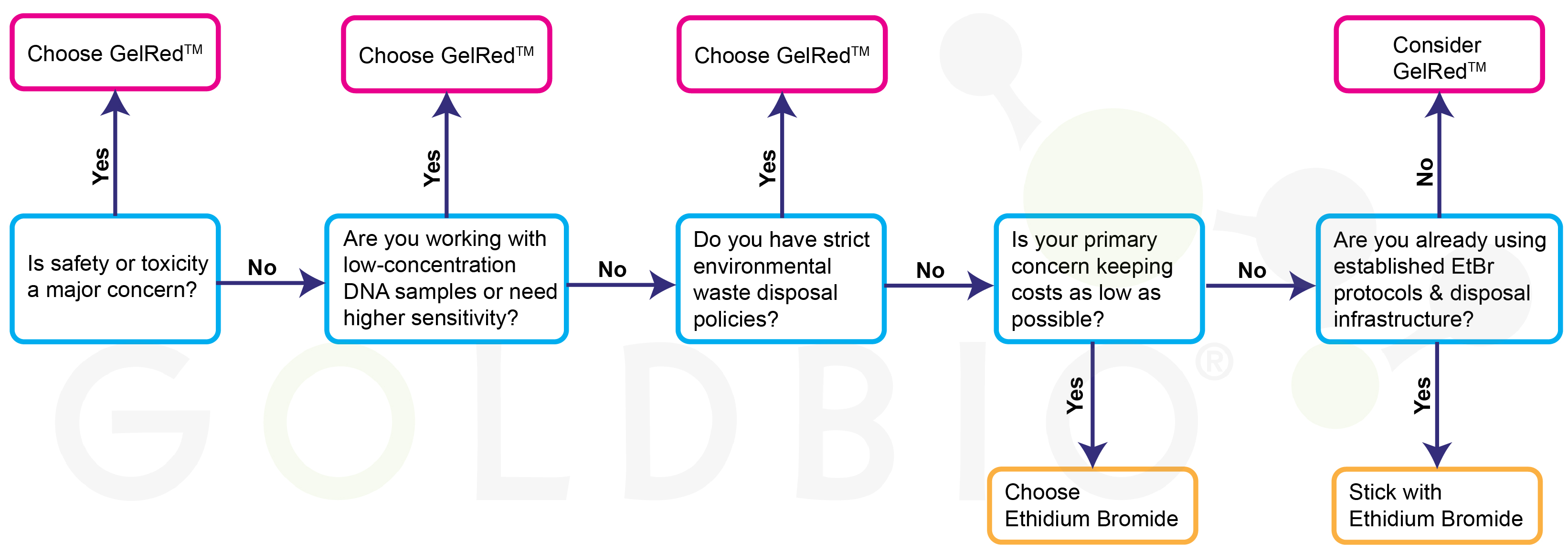
Figure 2. Decision map when choosing between GelRedTM
or ethidium bromide.
With many laboratories gradually transitioning to GelRedTM
and other EtBr alternatives as institutional safety policies evolve, now is a
good time to evaluate its use in your lab and get ahead of the curve.
Bitesizebio. (2025, February 12). Ethidium Bromide: The Alternatives. https://bitesizebio.com/417/ethidium-bromide-the-a...
Biotium. (2018, July 21). What is the binding mechanism of GelRed®/GelGreen®? https://biotium.com/faqs/gelred-gelgreen-binding-m...
Biotium. (2013). GelRed™ and GelGreen™ safety report: Summary of independent laboratory testing. Biotium, Inc. https://biotium.com/wp-content/uploads/2013/07/Gel...
Crisafuli, F. A. P., Ramos, E. B., & Rocha, M. S. (2015). Characterizing the interaction between DNA and GelRed fluorescent stain. European Biophysics Journal, 44(1), 1–7. https://doi.org/10.1007/s00249-014-0995-4
GoldBio. Top 10 GelRed Questions and Answers. https://goldbio.com/articles/article/top-10-gelredtm-questions-and-answers
Olmsted III, J., & Kearns, D. R. (1977). Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry, 16(16), 3647-3654. https://nathan.instras.com/MyDocsDB/doc-781.pdf
University of California, Santa Barbara. Ethidium Bromide: Swap or Not. https://www.sustainability.ucsb.edu/blog/just-fact...
University of Edinburgh. Ethidium Bromide Health & Safety Guidelines. https://health-safety.ed.ac.uk/guidance/hazardous-...
AI Disclosure: AI was used to both research and write significant portions of this article; however this article was deeply reviewed and edited by Simon Currie, PhD, and Katharine Martin.

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...

The GoldBio Floating Tube Rack is one of our more clever giveaways because of the unique purpose it serves. And, with it also being one...

The characteristic blue color of nickel agarose beads comes from the 2+ oxidation state of the nickel ions. Color is also a useful indicator for...
