Move Aside Dippin’ Dots™: the Next Ice Cream of the Future is Here
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
There’s nothing quite like cold ice cream on a hot summer day! The race is on to scarf down that delicious treat before it turns into a melted puddle. This is no problem for an experienced connoisseur like me, but for a newcomer like a young child, they’ve barely made a dent in it before it’s a melting mess.
New research from the University of Wisconsin is developing the next generation of ice cream, a version that resists melting (Wicks et al, 2023). The exciting thing about this research is that their modification makes the ice cream healthier for you too: two scoops all around!
The key to their “no-melt” ice cream is introducing a crosslinker – a molecule that binds the protein and fat molecules from the cream together (Figure 1). Conceptually, this is similar to crosslinkers used to stabilize agarose beads or crosslinkers that aid in studying protein-protein interactions, for example (Hoffman et al, 2015). You definitely wouldn’t want any of those kinds of crosslinkers in your ice cream, however, as they’re quite toxic.
Instead, the authors use polyphenol tannic acid (TA). You may have heard of polyphenols. They’re a type of small molecule found in vegetables and fruits, such as blueberries, and have been studied for their antioxidant and other health-promoting properties (Cosme et al, 2025).
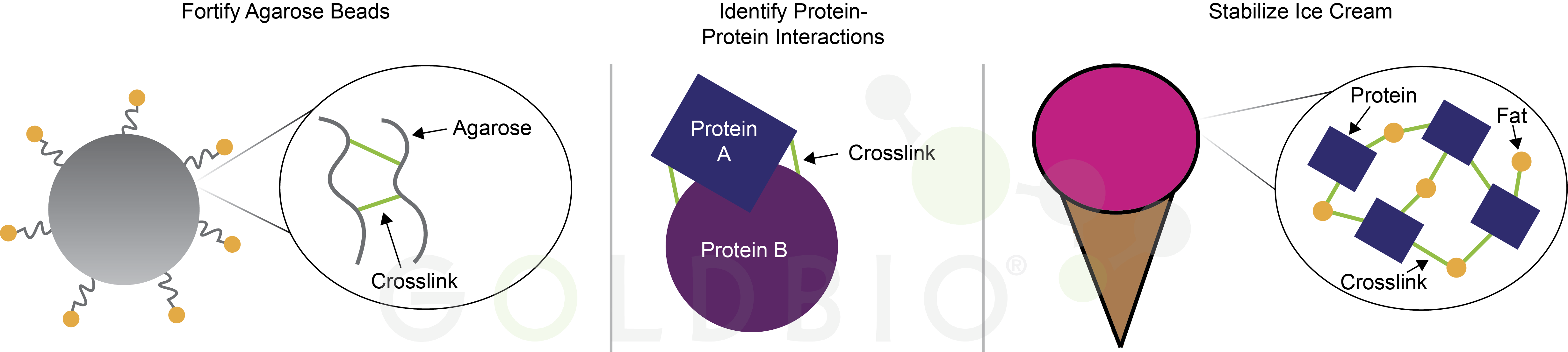
Figure 1.
Crosslinkers (green lines) are used to stabilize agarose beads (left), study
protein-protein interactions (middle), and stabilize ice cream (right).
Lead author Cameron Wicks, Ph.D., notes that commercial ice cream already uses binding agents such as guar and cellulose gums to minimize freezer burn during transportation. She suggests that replacing these ingredients with polyphenols may be attractive to ice cream consumers looking for a healthier option (Rottinghaus, 2024).
For her research, Wicks titrated polyphenol into ice cream and found that over the investigated range (0.75 – 3% of total weight) TA increased the viscosity of the ice cream and formed microscale fat globules. These are little pockets within the ice cream that are enriched in fat and protein molecules bound together by the TA crosslinks. While the TA crosslinks don’t bind to water molecules directly, the protein-TA-fat meshwork restricts the motion of the water molecules. So, even when the ice cream has “melted,” it retains its shape because the water molecules are still trapped within the fat globules (Figure 2). That is to say, even though the ice cream has melted at the molecular level, it doesn’t drip off of your cone.
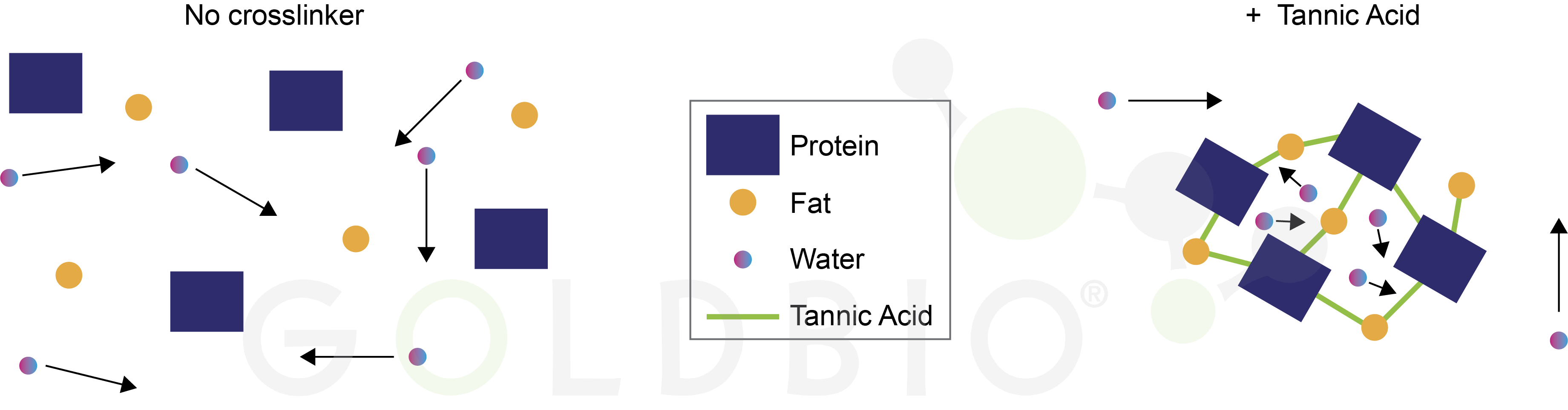
Figure 2.
Tannic acid crosslinks promote fat globule formation containing cream fat and
protein molecules and restricting the motion of water molecules. Arrows
indicate movement of water molecules.
To give a real-life analogy for this molecular phenomenon, let’s think about the kids’ game “Red Rover” where someone will try to run through a line of kids. The line of kids will all hold hands to try to prevent the runner from breaking through the line. Holding hands is just like crosslinks – it stabilizes the line of kids and makes them more resistant to the runner. At the molecular level, TA is crosslinking protein and fat molecules to make the ice cream more viscous.
Next, Wicks used important control experiments with SDS and EDTA to show that both fat and protein molecules are bound up in the fat globules. These globules slow down the melting rate of the ice cream because water molecules are also retained in them (Figure 2) (UW-Madison, 2024).
Wicks notes that dairy-free ice cream would be a next, more challenging application for this research which would require a different kind of innovation since it lacks the fat molecules that were key to forming the TA-mediated globules (Rottinghaus, 2024).
So, keep an eye out for polyphenol-containing, melt-resistant ice cream coming to a store near you soon. Alternatively, if you make your own ice cream you could experiment with adding in TA-containing blueberry powder to prevent your ice cream from melting on a hot day. Who knew science could be so tasty?
Cosme, F., Aires, A., Pinto, T., Oliveira, I., Vilela, A., & Gonçalves, B. (2025). A Comprehensive Review of Bioactive Tannins in Foods and Beverages: Functional Properties, Health Benefits, and Sensory Qualities. Molecules (Basel, Switzerland), 30(4), 800. https://doi.org/10.3390/molecules30040800
Hoffman, E. A., Frey, B. L., Smith, L. M., & Auble, D. T. (2015). Formaldehyde crosslinking: a tool for the study of chromatin complexes. The Journal of biological chemistry, 290(44), 26404–26411. https://doi.org/10.1074/jbc.R115.651679
Rottinghaus, M.L. (2024, Nov 12). How antioxidant-enhanced ice cream is changing the game for frozen treats. ASBMB. https://www.asbmb.org/asbmb-today/science/111224/how-antioxidant-enhanced-ice-cream-is-changing-the
UW-Madison. (2024, Jun 10). Bringing delight by investigating a no-melt ice cream [Video]. YouTube.
Wicks, C.J., Bolling, B.W. & Hartel, R.W. Effects of tannic acid on proteins and fat in cream. Food Prod Process and Nutr 5, 51 (2023). https://doi.org/10.1186/s43014-023-00166-9

The protein streptavidin clamps onto the small molecule biotin in one of the tightest natural interactions ever discovered. Over the years, the strength of this...

Maybe you are about to start working with D-luciferin, and you begin reviewing protocols. But what you’re doing is a little different. Or what you...

Handles are common in everyday life. We use them to help us open doors, cabinets, or to hold coffee mugs, cookware, and tools. They are...

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...
