Troubleshooting SDS-PAGE Sample Preparation Issues
by Pallabi Roy Chakravarty, Ph.D.

by Pallabi Roy Chakravarty, Ph.D.
There are five different signs that indicate that there are problems with SDS-PAGE sample preparation: sample leaking out of the well during or after loading, samples clumping and not migrating, you aren’t seeing bands, you’re seeing too many bands, or you’re getting smeared bands.
SDS-PAGE sample preparation issues:
In this article, we will discuss the issues that commonly occur and for each of these situations, the troubleshooting steps might solve the problem.
Samples leaking out of the well during or after loading
Samples clumping in the wells and bands not migrating properly
No bands are visible after staining the gel following electrophoresis
Too many bands in your gel after protein electrophoresis
General good practices for SDS-PAGE

Seeing distorted and smeared bands during protein electrophoresis can be due to samples leaking out of the well, which is caused by sample overload, uneven loading, incorrect buffers, or air bubbles in the wells during loading. Proper loading helps prevent issues and leads to better results.

Figure 1. Example of how sample leakage would appear during protein electrophoresis.
First possible explanation: The loading buffer may not have enough concentration of glycerol. Glycerol in your protein sample helps them sink down inside the well during loading.
Troubleshooting suggestion: Check for glycerol concentration in your loading buffer. You might need to increase glycerol concentration.
Second possible explanation: You might need to improve your gel loading approach. Loading samples in a polyacrylamide gel might not be as easy as loading an agarose gel for DNA electrophoresis. Below are some suggestions to keep in mind.
Troubleshooting suggestion: If there are air bubbles inside the well while you load that well, the sample would spill out during loading. The way around this is to first take a little bit of running buffer and rinse the well with it before loading your actual sample. This ensures that all air bubbles escape the well before you load your sample – this is an effective way to prevent samples from spilling out of the well during or after loading.
Another common issue, especially with people new to acrylamide gels, is that they tend to overfill the wells during loading. This potentially can lead to samples spilling out.
As a general rule, you would not want to load the well more than a maximum of 3/4 of its capacity.
Also, try to load all wells with equal volume.
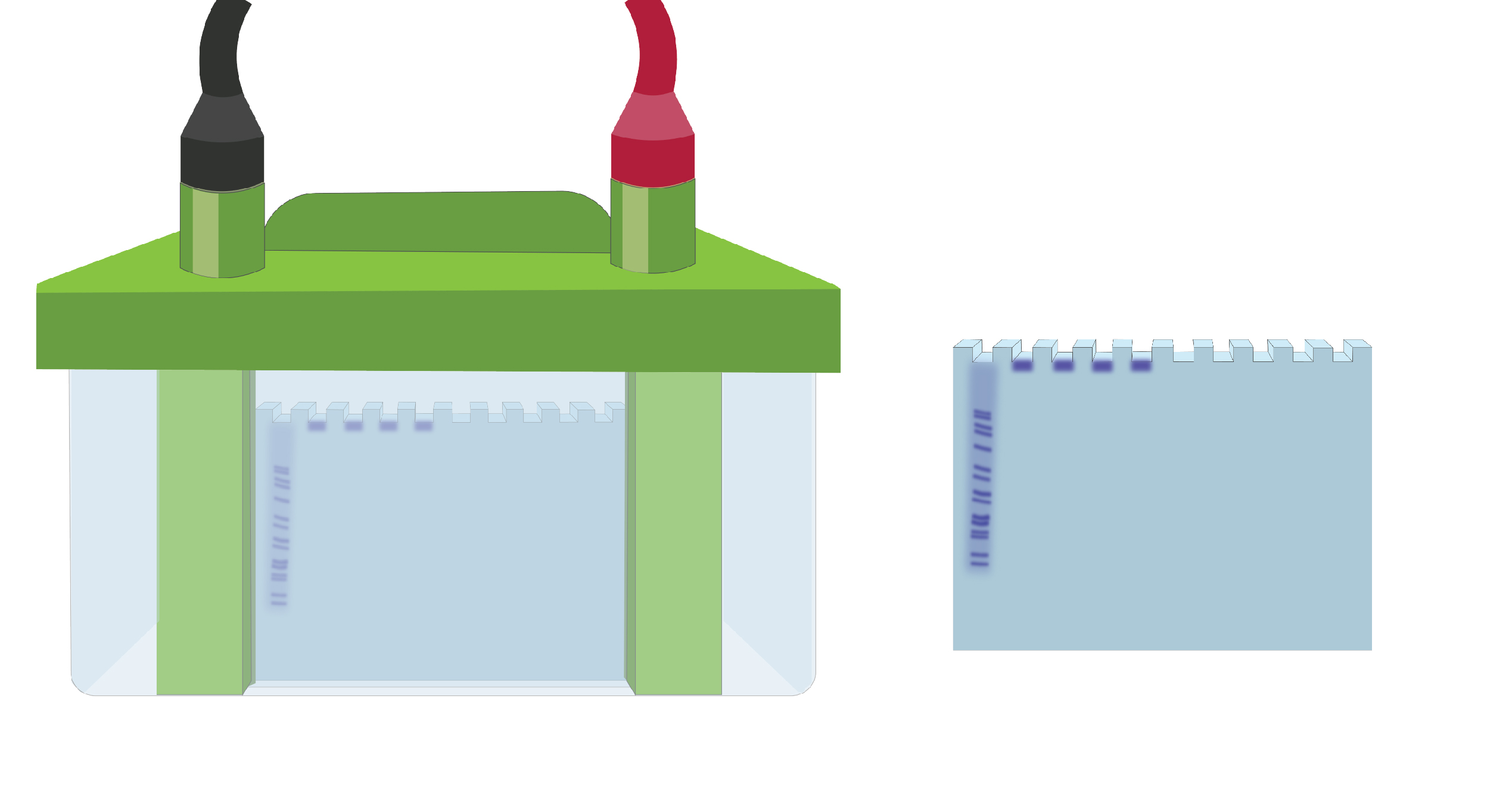
Figure 2. Illustration of what improper migration would look like.
First possible explanation: Too much protein was loaded in the wells.
Troubleshooting suggestion: Check the concentration of proteins in your samples. A good practice is to load 10 µg of protein per well.
If too much protein is loaded in the wells, band resolution will also usually be poor.
Second possible explanation: Protein aggregation or precipitation in the wells.
Troubleshooting suggestion: Ensure the solubility of your proteins in sample lysate before loading them into the gel. In this case, take extra care during the extraction of your sample proteins by doing proper homogenization.
One way of doing this is by sonicating your sample source (cell culture, bacterial culture etc.) adequately followed by centrifugation to remove cell debris.
Another suggestion is to add DTT or BME in your lysis solution. These chemicals reduce protein aggregation by breaking secondary structures.
Heating your lysate also aids in getting your sample proteins into their primary structure and reduces aggregation.
If your sample proteins are hydrophobic, there are higher chances of their aggregation. For this, consider adding 4-8M urea in your lysate solution before loading.

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...

The GoldBio Floating Tube Rack is one of our more clever giveaways because of the unique purpose it serves. And, with it also being one...

The characteristic blue color of nickel agarose beads comes from the 2+ oxidation state of the nickel ions. Color is also a useful indicator for...
