What is Forskolin? A Molecular Biologist’s Guide to this Powerful cAMP Modulator
by Katharine Martin

by Katharine Martin
Forskolin comes from the roots of the Coleus forskohlii plant. While it has many broader applications, it is also used across many different disciplines within molecular biology.
In molecular biology, forskolin is a labdane diterpene that binds to and activates adenylyl cyclase, which raises intracellular cAMP to modulate downstream cell signaling. Forskolin is used in cell signaling studies, hormone assays, GPCR studies and stem-cell differentiation.
But let’s take a closer look at what’s happening here. cAMP, also called cyclic adenosine monophosphate or cyclic AMP, plays a huge role in cell signaling as a second messenger (facilitates first messenger effects) in G protein-coupled receptor (GPCR) signal transduction.
Cyclic AMP is made when the enzyme adenylyl cyclase (AC) converts ATP to cAMP and pyrophosphate.
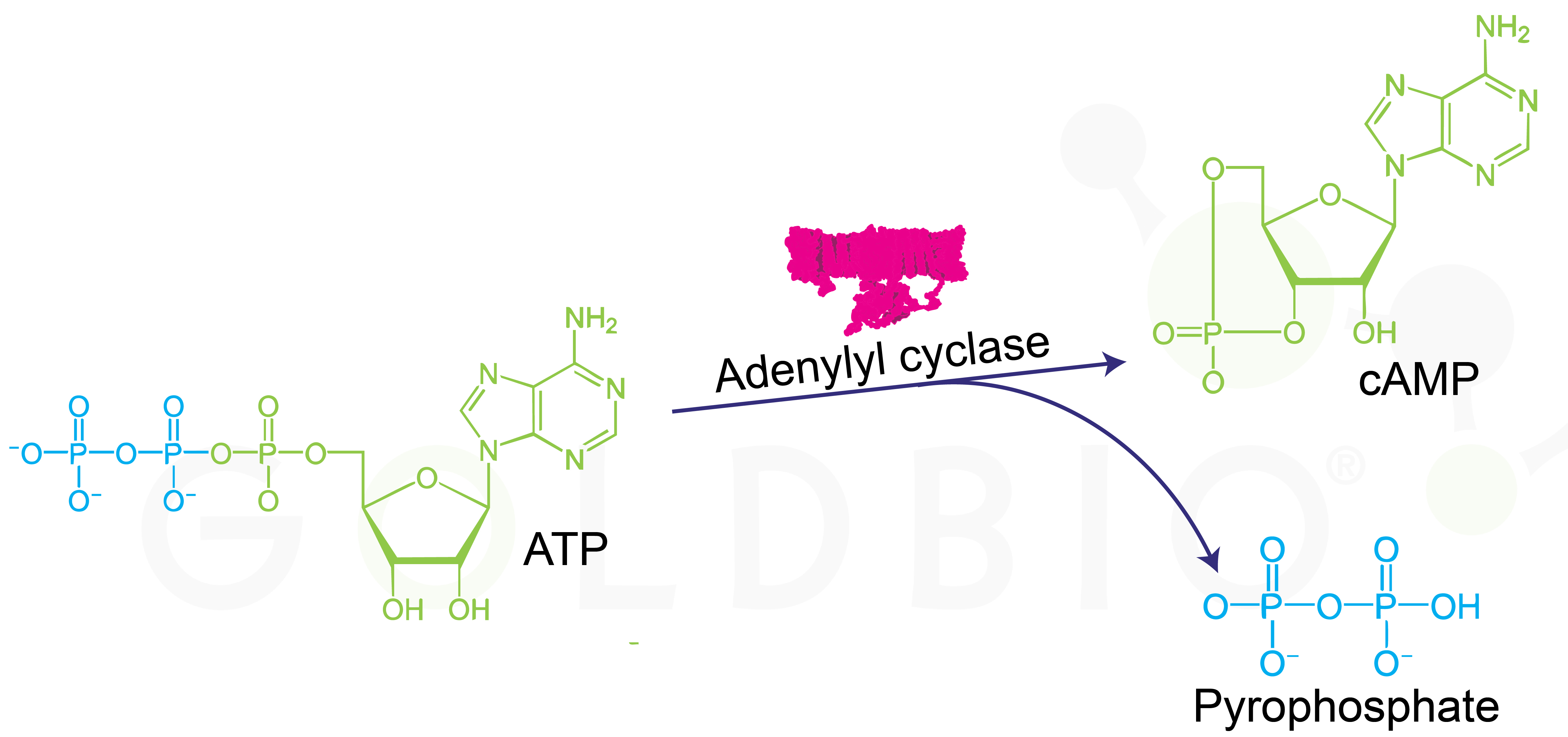
Figure 1. ATP to cAMP and pyrophosphate conversion
catalyzed by adenylyl cyclase (Mg
2+ cofactor not shown).
Forskolin activates adenylyl cyclase, which increases cAMP levels in many different types of cells. And since cAMP activates cAMP-dependent protein kinase A (PKA), which results in downstream cellular events, forskolin is important in initiating cell signaling studies.
In this article, we'll explore how forskolin works at the molecular level, examine the ways researchers are using it in their studies, and review common assays and applications.
How does forskolin modulate cell signaling?
How do researchers use forskolin in molecular and cell signaling studies?
What are some common applications using forskolin?
As we now understand, in the context of molecular biology, forskolin’s main role is to directly activate adenylyl cyclase, which increases levels of cellular cAMP. But how does it all work?
Let’s first look at the structure of adenylyl cyclase, also called adenylate cyclase. This enzyme has a catalytic subunit responsible for catalyzing the ATP – cAMP conversion.
The reaction’s catalysis requires free magnesium ions as essential cofactors. Under normal cellular conditions, adenylyl cyclase converts ATP into cyclic AMP.This process begins when Gs proteins are activated, causing their alpha subunit to bind to adenylyl cyclase, triggering catalytic activity.
Something to keep in mind is that there are different types of G proteins, and Gs proteins activate adenylyl cyclase, whereas Gi proteins inhibit its activity.
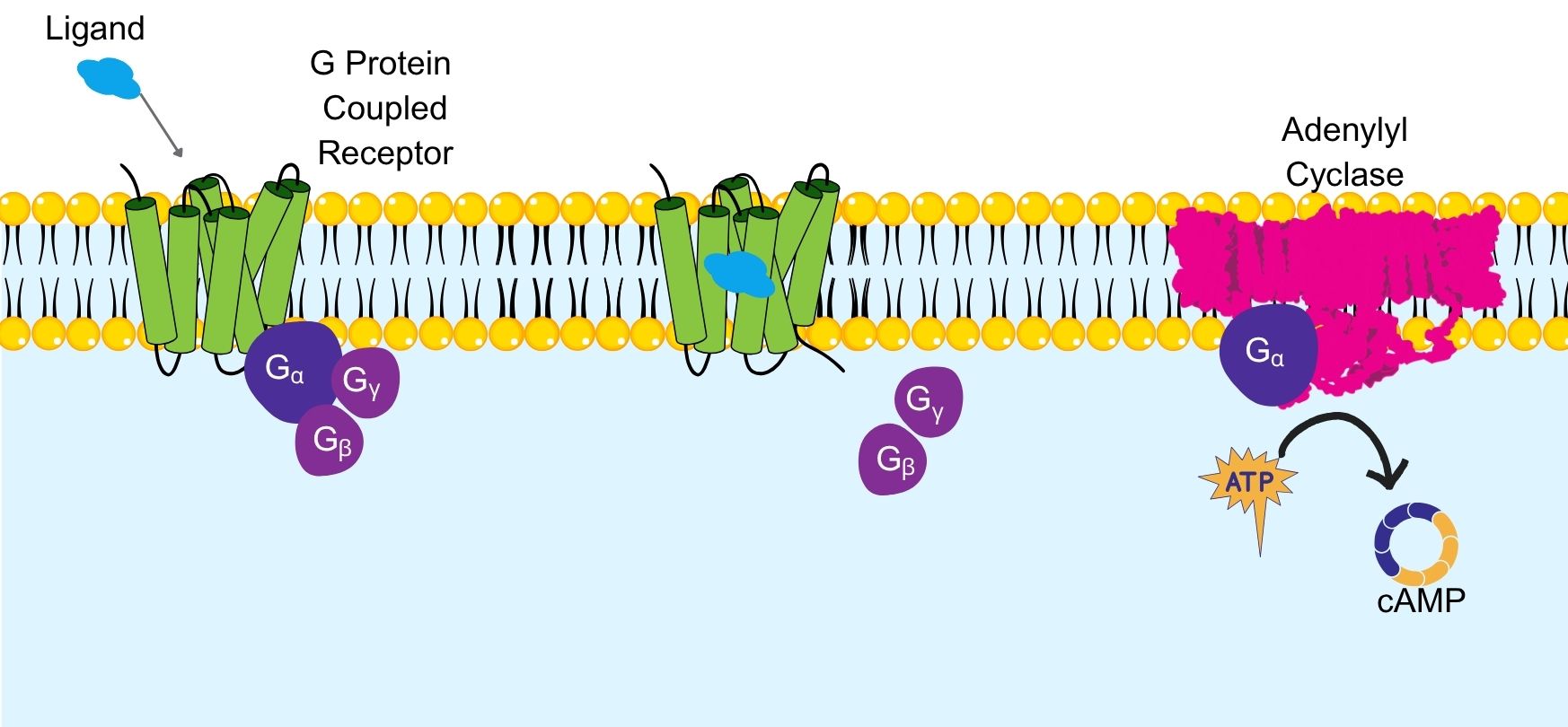
Figure 2. Simplified illustration of adenylyl cyclase
activation when a ligand binds to a G protein coupled receptor.
In this case, hormones or neurotransmitters would be responsible for binding to the cell surface receptors linked to G proteins, leading to a cascade of events
There are other molecules that can boost intracellular cAMP. These include prostaglandin E2 and adenosine. However, their effects on cAMP increases are indirect. Rather than binding to and activating adenylyl cyclase like forskolin will, these other molecules bind to G protein-coupled receptors (GPCRs), which indirectly increase cAMP levels.
The benefit of forskolin is that it bypasses GPCR signaling and allows receptor-independent cAMP increases.
When forskolin activates adenylyl cyclase, two forskolin molecules bind to adenylyl cyclase within a hydrophobic region. Once bound, forskolin helps maintain the geometry and stability of the C1a and C2a domains, allowing these domains to come together. When the C1a and C2a domains undergo pseudosymmetric dimerization (forming a functionally symmetric complex from structurally similar domains), they form the ATP binding and catalytic site containing the charged residues necessary for converting ATP to cAMP.
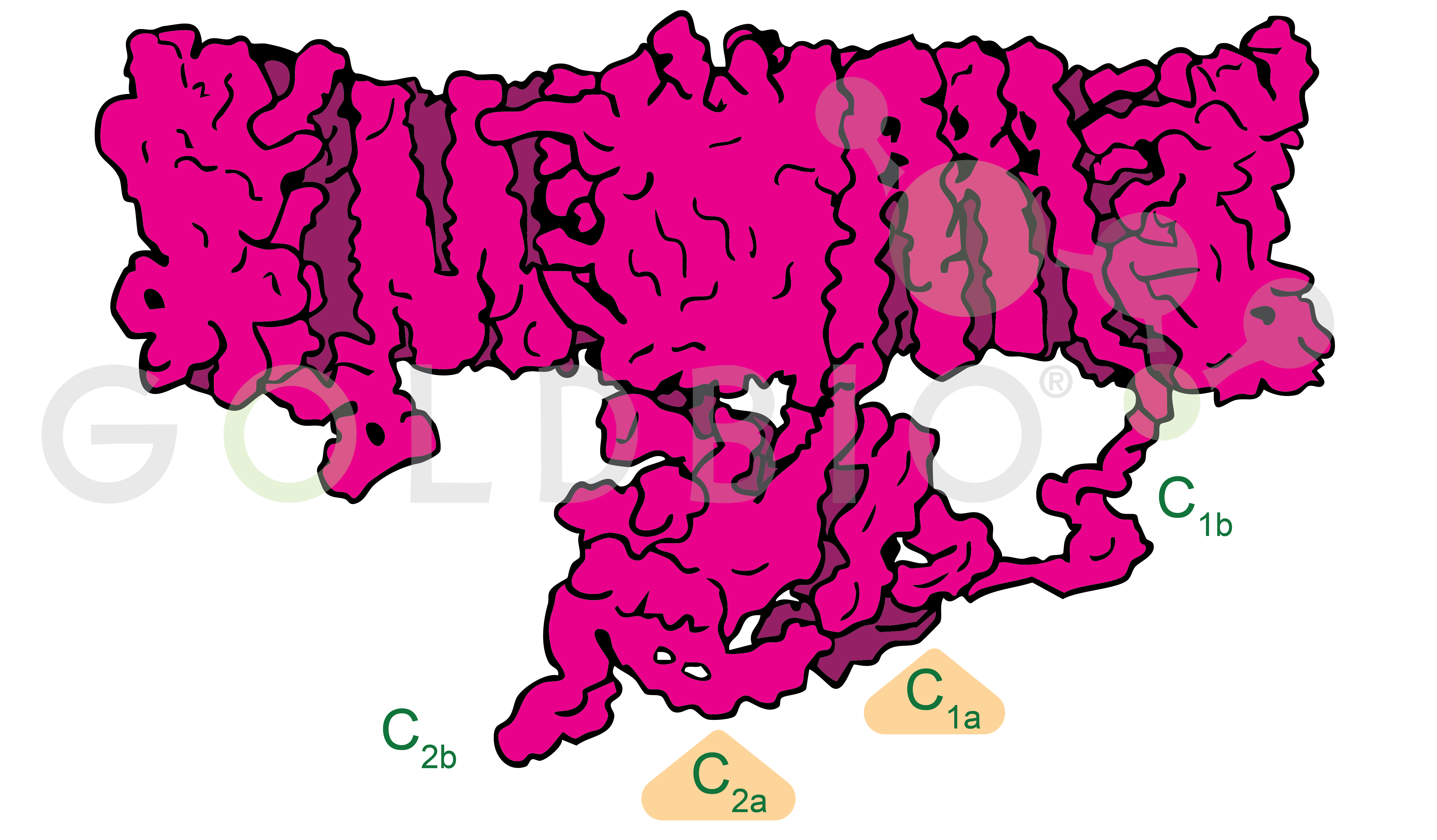
Figure 3. Cartoon illustration of adenylyl cyclase
with C2a and C1a subdomains labeled.
The direct activation increases cAMP levels, which then initiates
downstream cell signaling. This reliable and receptor-independent mechanism is
exactly what makes forskolin such a powerful research tool.
Because forskolin is directly involved in the increase of cAMP levels, it is an important component in cell signaling studies (PKA), for drug discovery (target validation), hormone feedback, synaptic plasticity, and stem cell differentiation.
There are numerous ways forskolin is used in molecular biology, but I will just point out a few.
In cell signaling, this study wanted to examine how Schwann cells influence inflammation. Within a microenvironment, researchers looked at two inflammatory pathways, one being the cAMP pathway, and used forskolin to activate the pathway. While this is just one example, it shows us that forskolin allows researchers to artificially induce the cAMP pathway for signaling studies (Henry, Wilcox, & Asirvatham, 2024).
Forskolin’s effects on cAMP increases the activity of a protein called cAMP-responsive element-binding (CREB), which promotes differentiation into neural cell types. It also promotes the regeneration of axons after injuries, making forskolin’s indirect effects on cell differentiation a valuable research tool.
For instance, Singh, Vaishnav, Dinda and Mohatany used forskolin in combination with growth factors and media supplements to induce neuronal differentiation (2020).
The forskolin-induced swelling assay is another interesting use case. This assay is used to measure the individual function of cystic fibrosis transmembrane conductance regulator in organoids. The reason this assay is important is because the CFTR gene mutation that causes cystic fibrosis has over 1,900 mutations.
In this assay, forskolin induces organoid swelling, which is then measured, allowing researchers to determine the activity of CFTR channels. These examples demonstrate forskolin's versatility across different research contexts, from basic cell signaling studies to clinical applications.
Because of its role in modulating cAMP activity, forskolin is used in a wide range of applications and assays including:

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...

The GoldBio Floating Tube Rack is one of our more clever giveaways because of the unique purpose it serves. And, with it also being one...

The characteristic blue color of nickel agarose beads comes from the 2+ oxidation state of the nickel ions. Color is also a useful indicator for...
