What’s the Binding Capacity of Glutathione Agarose
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
You want to purify Glutathione S-transferase (GST) or a GST-fusion protein. So, you pull your GoldBio glutathione agarose beads out of the refrigerator to start purifying your protein. Right then it hits you: “how much of this am I supposed to use?”
Like other affinity purification techniques, it’s important to use the proper amount of agarose beads to capture as much of your target protein as possible. Yet, you don’t want to use too much glutathione resin and waste your lab’s precious resources.
The binding capacity of glutathione agarose is approximately 8 milligrams (mg) of GST-tagged protein per milliliter (mL) of resin. The exact binding capacity will depend on a number of parameters including binding buffer and the size of the GST-fusion protein.
In this article, we’ll cover how to estimate an appropriate amount of glutathione resin to use based on your specific protein and purification protocol.
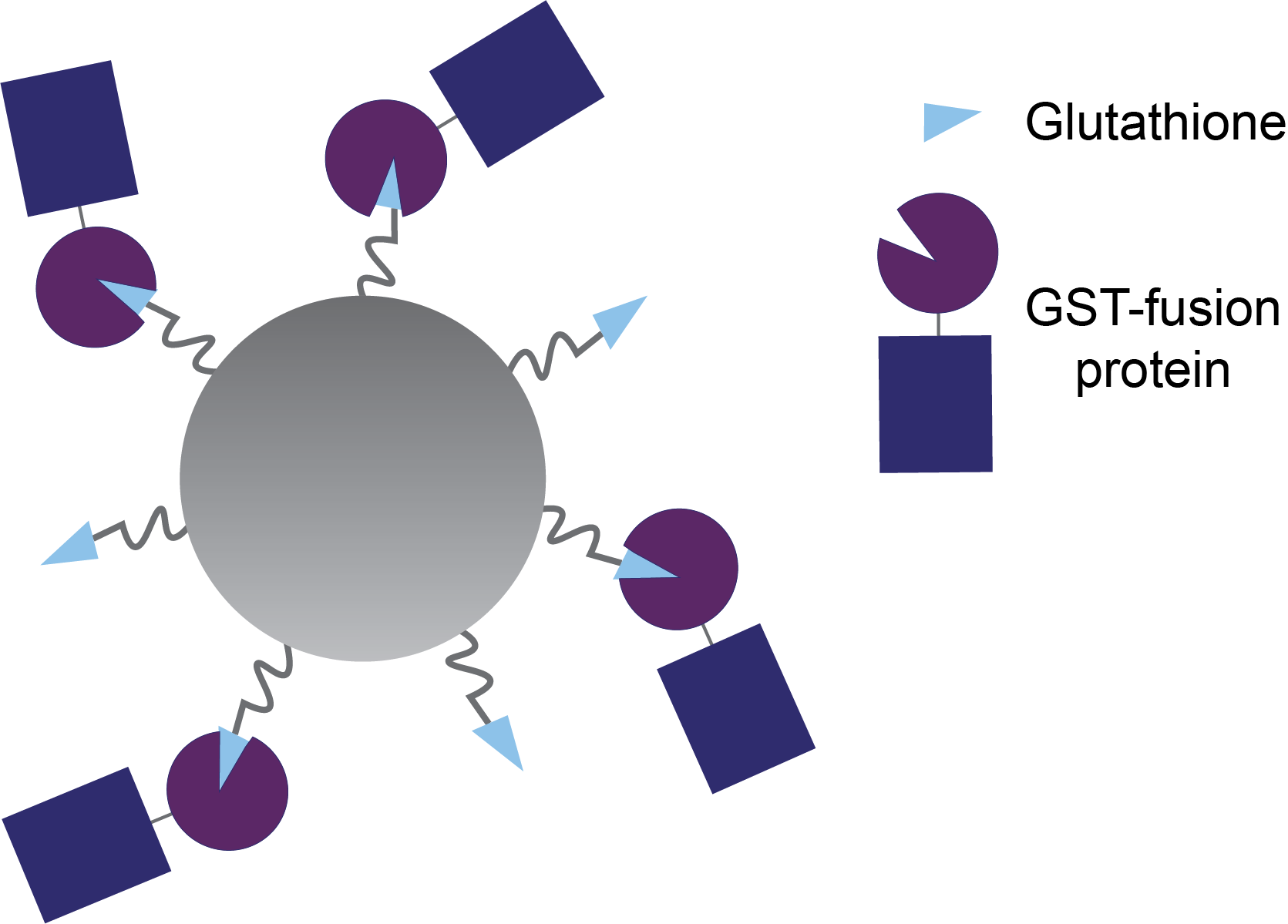
Figure 1. Glutathione agarose beads with GST-fusion proteins bound.
What is a mL of glutathione resin?
How does the size of my protein impact binding capacity?
Estimating volume of glutathione agarose needed
First, we want to start with understanding the context of a milliliter when it comes to binding capacity. So, let’s make sure we’re on the same page about what a mL of glutathione resin is.
GoldBio glutathione agarose resin comes as a 75% (v:v) aqueous suspension in 20% ethanol. That means if you pipet out one mL of resuspended resin, there will only be about 0.75 mL of resin in there. So, you’ll want to pipet out 1.33 mL of resuspended resin for each 1 mL of glutathione resin that you’re using for your purification.
The Math:
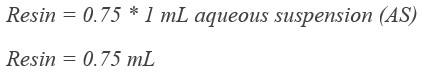
We want 1 mL of our glutathione resin...
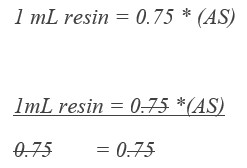
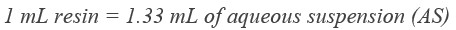
What is resuspended resin? Resuspended resin just means that you want to invert the bottle of agarose resin a few times to make sure that all the beads are off the bottom of the bottle and in solution before you pipet some out of there.
Choosing a good binding buffer is key to making sure that you’re capturing as much of your GST-fusion protein as possible. In our Affinity GST Purification Protocol, we recommend using 1x PBS, pH 7.3 as the binding buffer.
Really, a wide range of buffers will work just fine here; you just want to make sure that you avoid anything that will disrupt the glutathione-GST interaction that binds your protein to the beads. Most neutral pH buffers work well. Just remember to save the free glutathione for your elution buffer!
If you need more suggestions about buffer conditions, check out this handy chemical compatibility chart for our glutathione agarose beads.
Your choice of binding buffer will influence the binding capacity of the beads, so if you are capturing substantially less than 8 mg of protein per mL of resin, and your protein isn’t really big, then it is worth considering if your binding buffer is limiting the capture efficiency of the beads.
The bigger your GST-fusion protein is, the lower the binding capacity will be (Figure 2).
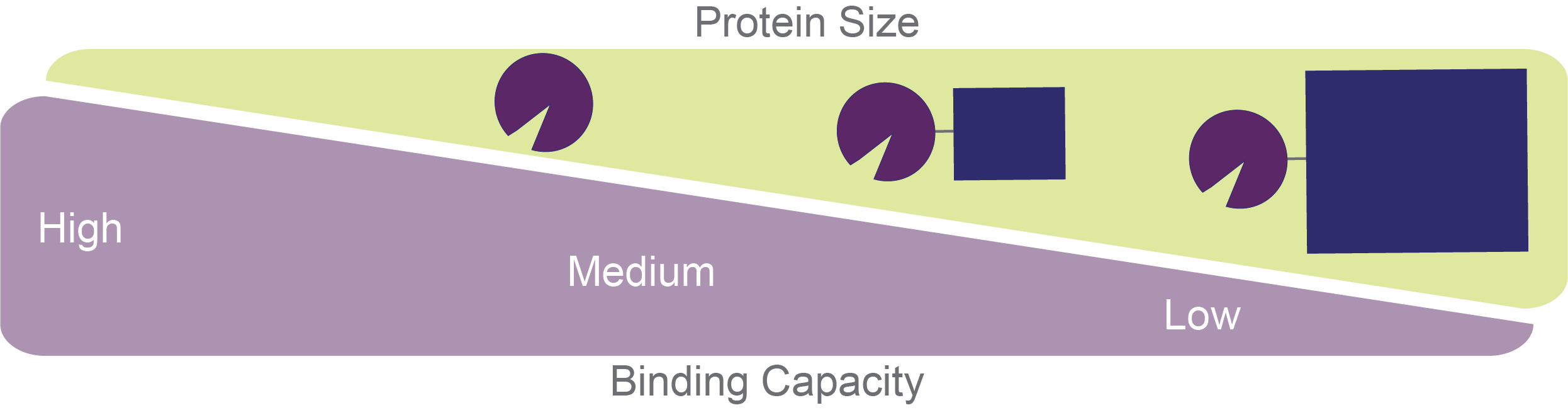
Figure 2. Tradeoff
between protein size and glutathione agarose binding capacity. GST by itself
will have pretty high binding capacity, whereas the larger the protein of
interest that is fused to GST is, the lower the binding capacity will be.
To think about why that is, let’s first consider an analogy. How many items could you catch in a baseball glove? That depends on what the item is, right? You could probably fit a dozen golf balls, three to four baseballs, two softballs, or one volleyball into the mitt. The larger the item, the fewer will fit into the same space.
It’s a similar idea with binding to glutathione agarose beads. There are many glutathione molecules conjugated to each agarose bead, so multiple GST-fusion proteins can bind to the same bead (Figure 3).
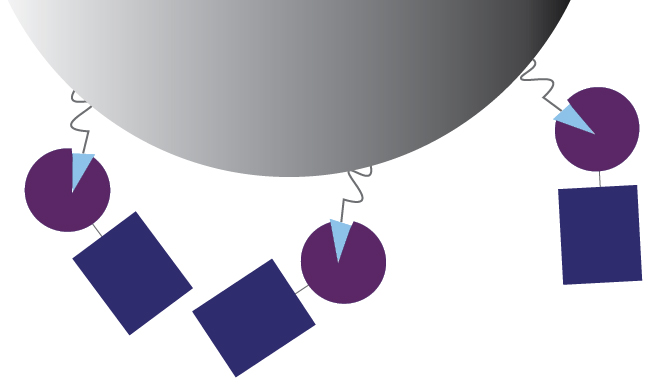
Figure 3. With
smaller GST-fusion proteins, several proteins can bind to glutathione ligands
on the same agarose bead. The little blue rectangles are the proteins of
interest, fused to GST (purple).
But larger GST-fusion proteins mean fewer can bind to each
bead at the same time without running into one another (Figure 4).
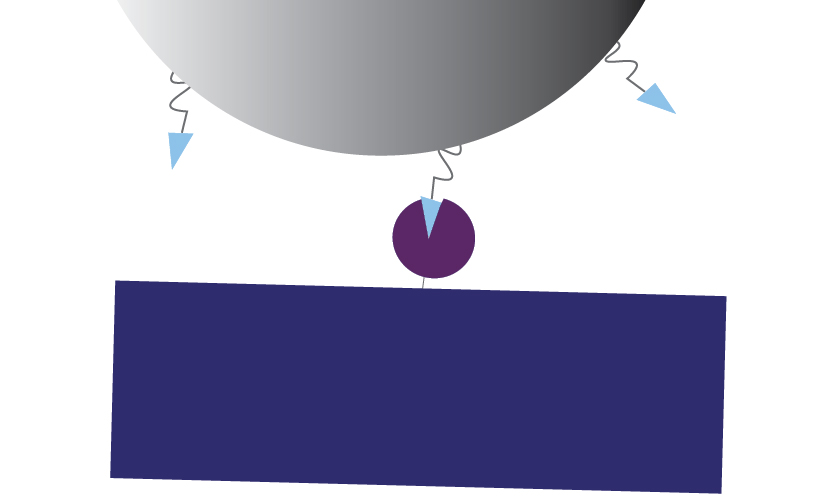 Figure 4. Larger GST-fusion proteins sterically block each other from binding to proximal glutathione ligands. Large blue rectangle is the protein of interest fused to GST (purple).
Figure 4. Larger GST-fusion proteins sterically block each other from binding to proximal glutathione ligands. Large blue rectangle is the protein of interest fused to GST (purple).
Those are the factors that influence glutathione binding capacity. If your purifications are yielding close to 8 mg of GST-fusion protein per mL of glutathione resin, you are good to go. But, if your yields are substantially lower than that, and you’re not working with a big GST-fusion protein, then it is worth considering how you can optimize the binding buffer during the capture step.
Ok, so we’ve discussed a lot about what factors impact binding capacity which will influence the volume of glutathione agarose you should use. But how do you actually estimate that volume? Let’s work through a hypothetical example.
First, you need to have an estimate of how much of your protein you’re purifying. If this is your first time purifying this protein, your estimate might not be great. But don’t worry, you can always check afterwards to see how good this initial estimate was.
If you have purified the protein before, you would already have a reasonable estimate of the starting amount of protein.
For the purposes of this exercise, let’s suppose that you’re starting with 100 mg of GST-fusion protein from one liter of fermented culture.
Let’s say that your target protein is smaller than GST, such that most of the size of the GST-fusion protein will be contributed from GST and not your target protein. In that case, you would expect the binding capacity of your target protein to be close to the reported 8 mg per mL of resin.
You’re using 1x PBS, pH 7.3 as your loading buffer without any additional reduced glutathione added. So again, you expect the binding capacity to be near the ideal 8 mg per mL of resin.
So, to figure out how much resuspended resin you need to pour out of the bottle and into your column, you just need to make two quick calculations.
If you want 100 mg of protein, then we’ll start with that number: 100 mg. And each mL of glutathione resin captures 8 mg of GST-fusion protein, so you’ll divide 100 mg by the binding capacity of 8 mg per mL of resin to calculate how much total resin you need. 100 divided by 8 is 12.5, so you need 12.5 mL of glutathione resin.
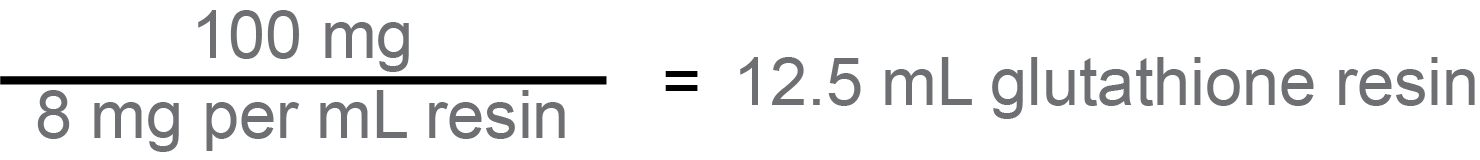
Second, to account for the buffer that is in the resuspended 75% (v:v) aqueous suspension, you multiply 12.5 mL by 1.33, as we discussed above, which equals approximately 16.6 mL of resuspended resin that you need to use.

Is this a good estimate of how much glutathione agarose you should use? Especially if this is your first time purifying this protein, your estimate of having 100 mg of protein might be a bit off. By quantifying how much of your protein was in your elution, and analyzing how much, if any, of your protein was in the flow-through, you can figure out if you used an appropriate amount of glutathione resin.
For our first scenario, let’s say that the final yield of your elution is 10 mg of GST-fusion protein. This is much less than the 100 mg we were hoping for. Assuming you followed the above advice and used an appropriate binding buffer and your protein isn’t huge, then a likely reason for this is that the expression yield is much lower than you expected.
In this scenario, you would expect all of your protein to be in the elution, and none of it to be in the flow-through. Next time, if this happens, you could either scale up your expression tenfold, or you could use the same expression combined with approximately ten times less glutathione resin.
In the second scenario, the final yield of your elution is close to 100 mg of GST-fusion protein, so you’re feeling confident that you used the right binding buffer and that your protein isn’t too big. But did you get all the protein that you could have? It’s definitely worth checking on an SDS-PAGE gel to see whether you should use more glutathione resin next time.
If none of your protein, or very little, is in the flow-through during your purification, then you’ve used sufficient glutathione agarose (Figure 5, left).
Alternatively, if there is still quite a bit of your protein in the flow-through then you could use even more resin next time to capture more of your target protein (Figure 5, right).
Of course, 100 mg of protein is a lot for most applications so you may be fine with that yield and not care about losing some of your protein in the flow-through, depending on what the downstream application is for your protein. But in either case, it is always worth analyzing your purifications to know if there is any room for further optimization.
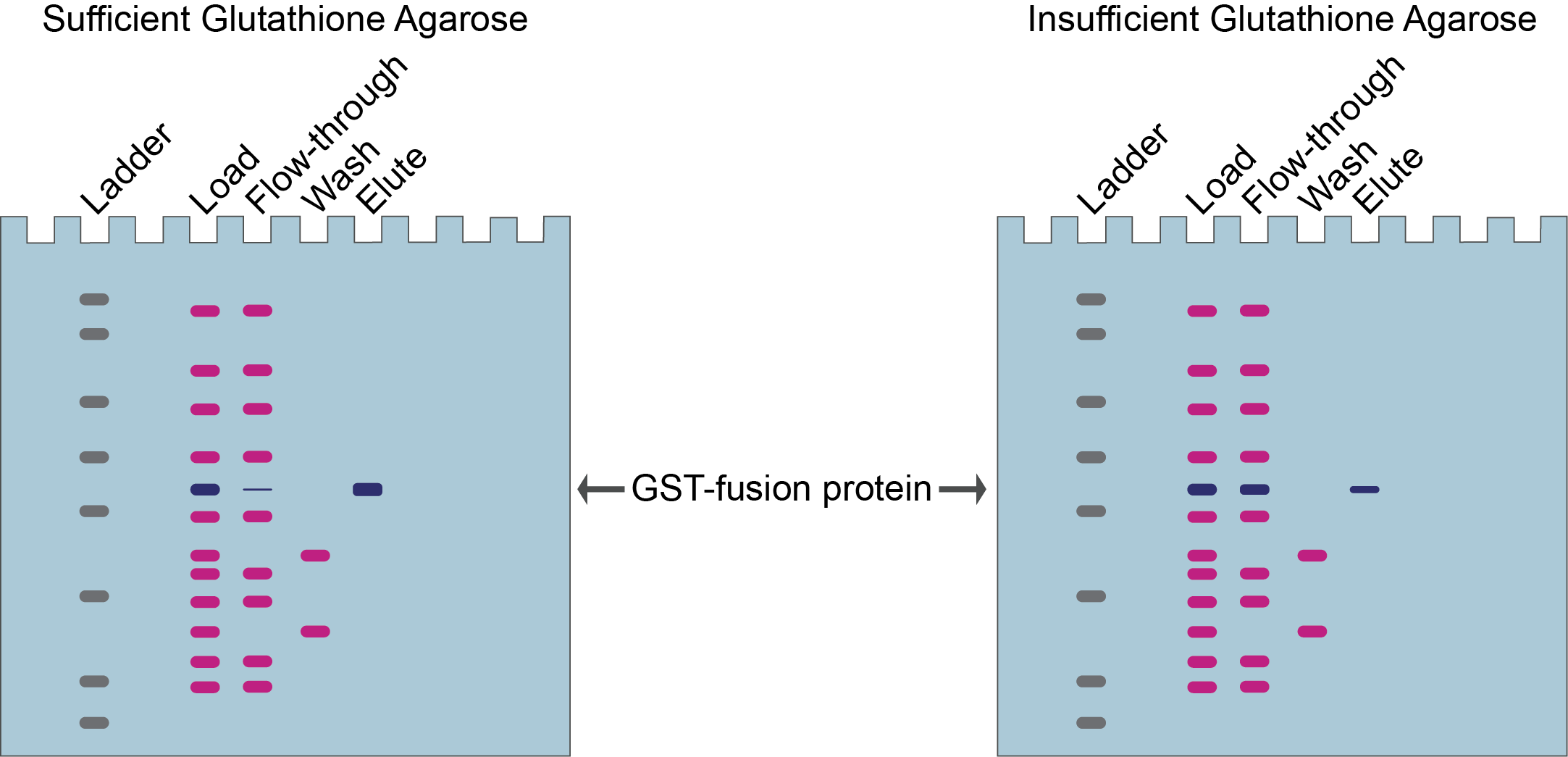
Figure 5. Left gel shows very little GST-fusion protein in the flow-through and a lot in the elution indicating that sufficient glutathione agarose resin was used in this purification. Right gel shows a lot of GST-fusion protein in the flow-through indicating that insufficient glutathione agarose resin was used for this purification.
That’s a quick overview of how to estimate how much agarose resin you should use based on the binding capacity, protein of interest, and purification conditions. For more details on purification with glutathione resin, check out this detailed protocol and troubleshooting guide.

IPTG and auto-induction are two ways to induce protein expression in bacteria. They work similarly, but have different trade-offs in terms of convenience. While IPTG...

The final concentration of IPTG used for induction varies from 0.1 to 1.0 mM, with 0.5 or 1.0 mM most frequently used. For proteins with...

A His-tag is a stretch of 6-10 histidine amino acids in a row that is used for affinity purification, protein detection, and biochemical assays. His-tags...

Competent cells such as DH5a, DH10B, and BL21 will maintain their transformation efficiency for at least a year with proper storage. It is important to...
