Transduction: P1vir Phage Overview - the mechanisms, characteristics and role in transduction
by Pallabi Roy Chakravarty, Ph.D.

by Pallabi Roy Chakravarty, Ph.D.
Transduction for genetic engineering of bacteria, over the years, has been standardized using the model bacterium E. coli and the bacteriophage P1. This has resulted in P1 becoming a very well-characterized tool.
To facilitate optimal transduction of a DNA fragment of interest from one bacterial strain to another using this bacteriophage system, scientists have devised numerous innovations in the experimental procedure. The most important among these is creating a mutant of P1 called P1vir, and using that in place of P1 for transduction.
Why P1vir is used instead of P1 for transduction
Important characteristics of the P1vir phage transduction system
Importance of the selectable marker in the P1vir transduction process
How is the fragment of interest engineered in the host bacterium for transduction to the recipient?
Characteristics necessary in the recipient cell for P1vir transduction
Scientific improvisations for successful P1vir transduction
The lytic cycle is required for transducing the fragment of interest from the host bacterium’s genome into the recipient bacterial cell.
However, the P1 bacteriophage is temperate, meaning it can infect by both lytic and lysogenic cycles. To restrict the infection cycle exclusively to the lytic form, a vir mutation has been made by scientists creating the phage variant P1vir.
This P1 variant can infect only through the lytic cycle. P1vir, rather than P1, is the actual bacteriophage used regularly in the lab for transduction procedures.
Using P1vir as an example, we shall discuss how a typical transduction procedure is performed in a laboratory setting.
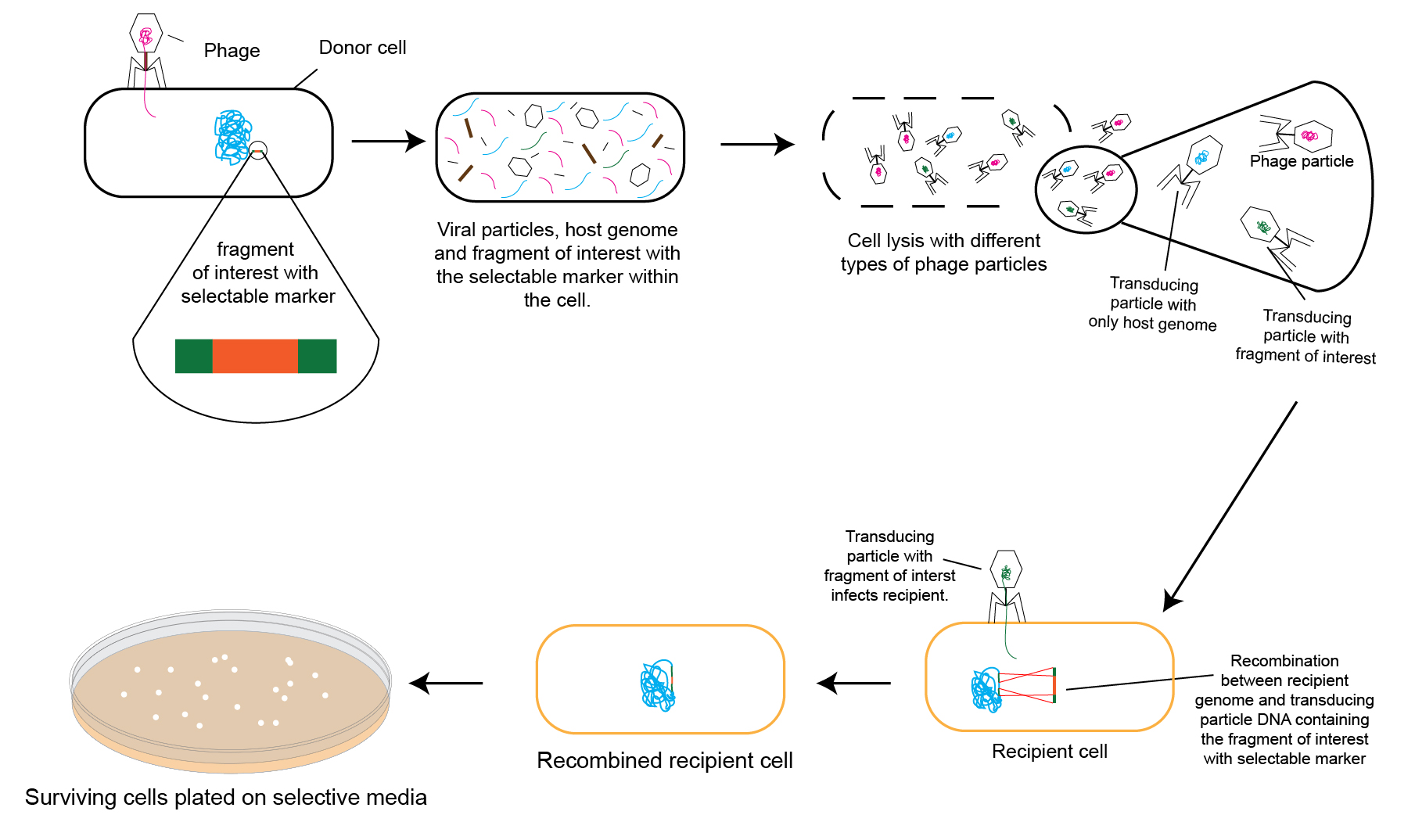
Figure 1 below schematically describes a typical bacterial transduction method.

Figure 1 Schematic diagram of a typical transduction procedure in the laboratory for genetic manipulation of bacteria.
For greater clarity, each part of the above figure is described in snippets below.
The donor bacterial cell has a selectable mutation (commonly made using Lambda Red Recombineering) in its chromosome that needs to be transduced to the recipient (as shown in figure 1). This donor cell is infected with the bacteriophage, which will eventually lead to donor cell lysis.

While most phage particles coming out of the lysed donor cell pack their own genetic material in their capsids, a few will pack parts of the donor cell’s chromosome. The latter are known as transducing particles.
A small proportion of these transducing particles carry the selectable mutation from the donor’s chromosome. Note that in the following snippet of figure 1 the transducing particles are represented in green and blue.
The green transducing particle contains part of the host bacterial chromosome that has the selectable mutation, and the blue transducing particle contains part of the host bacterial chromosome that does not have the selectable mutation.

When recipient cells are infected with the lysate from the donor, they may be infected by one of three ways:
A) The first way is by a transducing particle carrying the selectable mutation that is desired to be transduced. In this case, this selectable mutation is transduced into the recipient cell’s chromosome. When plated on selective media, an antibiotic plate for example, this cell (now a transductant) survives to produce colonies owing to the transduced selectable marker (antibiotic resistance cassette). The figure 1 snippet below helps illustrate this concept.

B) The second way is for a transducing particle that carries part of the donor’s chromosome (shown in blue) other than the desired selectable mutation. In this case, though this recipient cell will be a transductant, it will die when plated on selective media because it does not have the selectable marker (antibiotic resistance, in this case) needed to survive the selection pressure.

C) In the third case of infection, the phage carries its own genome instead of packing parts of the donor bacterium’s genome. In this scenario, the lytic cycle will commence leading to the recipient cell’s lysis.

DNA fragments of interest of up to 100 kb can be packed in its capsid and transferred from one strain to another.
P1vir has been engineered to infect bacteria exclusively through the lytic mode. This is critical to ensure transduction of the DNA fragment of interest from the host cell’s chromosome to the recipient cell.
As shown in figure 1, the lysate produced by infecting the host bacterium with P1vir contains these two types of particles: P1vir phages, and transducing particles. With transducing particles, part of the host bacterial chromosome is packed in the phage capsid instead of the phage’s genome. In the lysate, 0.1% are transducing particles. The rest are P1vir phages. Of the transducing particles,0.1-1%are estimated to harbor the fragment of interest from the donor’s chromosome.
Fragments that are transmitted from the donor to the recipient strain need to be associated with a selectable marker for positive selection of the required transductants.
Figure 2 demonstrates the importance of the selectable marker in the process of transducing a desired chromosomal fragment of interest from the donor bacterium to the recipient.
Remember that when our donor cell was first infected with the phage, there were three different types of phage particles that would be present in the donor cell lysate . One of these types of particles are phage particles (pink). This constitutes the first type of particles in the donor lysate.
The other two types of phages in the donor lysate are transducing particles (green and blue).
Of the two types of transducing particles (green and blue phages), figure two shows the process that occurs with the transducing particle that contains the fragment of interest with selectable marker (green).
The second type of transducing particle in the donor cell lysate are ones which pack part of the host cell’s chromosome other than the locus having the selectable marker.
When these phage particles infect the recipient strain, they indeed produce transductants (shown above). However, with the absence of the selectable marker, such transductants would not be able to grow on the selection plate. Thus, without a selectable marker, transductants cannot be differentiated from non-transductants.
Please note that the donor strain lysate contains phages that pack random parts of the donor’s chromosome (transducing particles shown in blue in the figures above). These phages induce transduction of other parts. Such fragments, once transduced into the host, recombine with their corresponding homologous regions in the host chromosome. These are non-desired transductants. And because these do not have the selectable marker, they fail to grow on the selection plate. Therefore, using a selectable marker such as an antibiotic resistance cassette, desired transductants can be differentiated from and selected over undesired transductants.
Fragments typically transduced are genes that have been substituted with an antibiotic resistance cassette.
Another example of how a fragment of interest is engineered for transduction into the host is as follows. In this case, a gene locus in the host cell’s chromosome needs to have a point mutation made, rather than a full deletion as in the previous example above.
Such a point mutated allele is first engineered in the donor’s chromosome, most commonly using Lambda Red Recombineering. Further, an antibiotic resistance marker is introduced into the donor’s chromosome to closely flank this mutated allele. Whenever that mutant allele is transferred via transduction, the linked selectable marker is also co-transduced to the recipient simultaneously.
Lambda Red Recombineering is commonly used to create these selectable mutations in the host bacterial strain. Once suitably mutated using Lambda Red, the host strain is infected with P1vir to transduce that mutation to a suitable recipient bacterial strain.
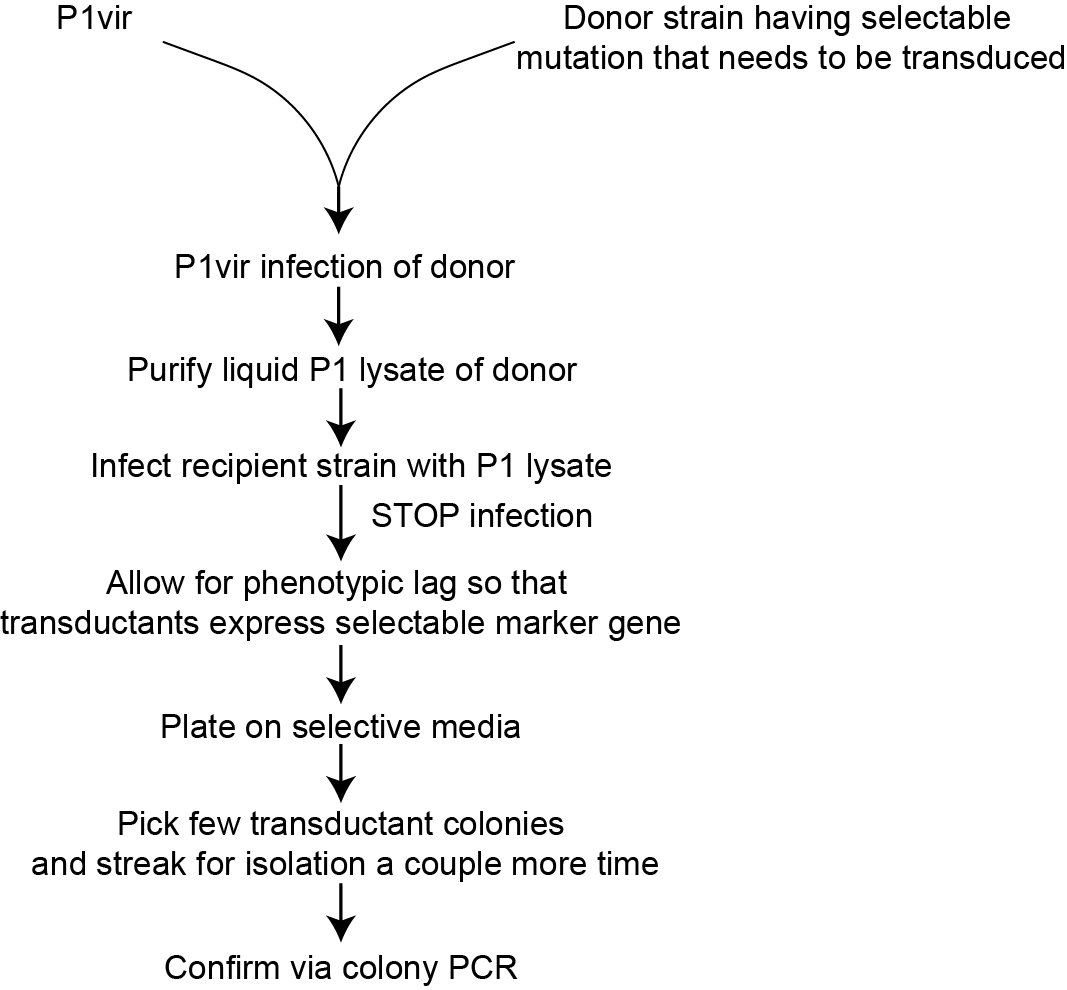
Figure 3. A schematic workflow of P1vir transduction.
The first characteristic is that the recipient bacterial cell cannot be a recombination mutant, that is, a cell that lacks adequate machinery for genetic recombination.
The reason this is important is because once the transducing particle injects the donor’s genetic element into the recipient cytoplasm, the donor’s recombination machinery is required for incorporation of the fragment into its chromosome.
Another important characteristic of the recipient cell has to do with the presence or absence of a specific sugar residue. The P1vir bacteriophage attaches to a specific sugar residue on the host cell’s surface. Bacterial cells lacking this sugar residue on their surface cannot be transduced by this method. However, bacterial cells with the sugar residue present, can be transduced.
Scientists, over the years, have devised ways to optimize transduction using the P1 bacteriophage as a tool. One critical improvisation is the mutation of P1 to P1vir. Some other experimentalconsiderations for successful transduction using this method are described in the following sections.
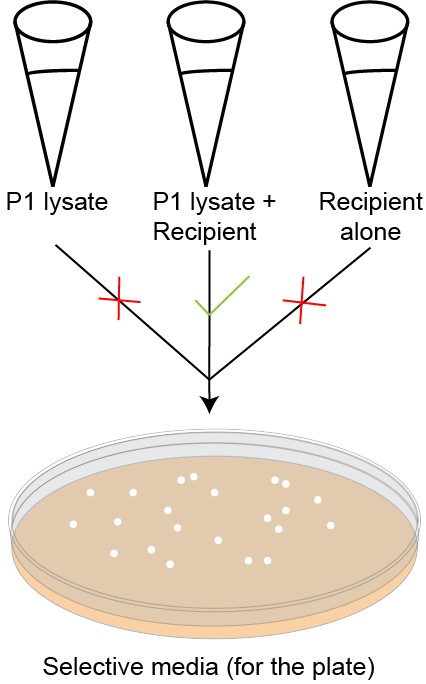
Figure 4. Controls to monitor validity of P1vir transduction. All colonies showing on the selective plate are a result of the P1vir+ recipient (second tube). P1vir lysate should not produce colonies on the selection plate. P1vir lysate+ recipient should produce colonies of transductants if the transduction has worked. If the recipient alone is plated, there should not be any colonies on the selection plate.
In this article we learned how genetic mutations can be effectively transmitted from one bacterial strain (donor) to another (recipient) using transduction with P1vir as a classic example. To put things into a holistic perspective, the next question to answer is: how such mutations can be made in the donor bacteria’s chromosome in the first place?
Thomason L.C et al, 2007. E. coli genome manipulation by P1 transduction. Current protocols in molecular biology. Chapter 1:Unit1.17. doi: 10.1002/0471142727.mb0117s79
Thierauf A et al, 2009. Generalized transduction. Methods in molecular biology. 501:267-86. doi: 10.1007/978-1-60327-164-6_23

The protein streptavidin clamps onto the small molecule biotin in one of the tightest natural interactions ever discovered. Over the years, the strength of this...

Maybe you are about to start working with D-luciferin, and you begin reviewing protocols. But what you’re doing is a little different. Or what you...

Handles are common in everyday life. We use them to help us open doors, cabinets, or to hold coffee mugs, cookware, and tools. They are...

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...
