Genome Editing Validation using T7 Endonuclease I
by Fernanda Ruiz, PhD

by Fernanda Ruiz, PhD
T7 endonuclease I is a popular and very useful enzyme that is often used to quantify a targeted mutation. Here, we will describe the mechanism of this powerful nuclease and guidelines for its use in genome editing.
Powerful genome editing technologies like CRISPR, transcription activator-like effector nucleases (TALENs) and meganucleases zinc finger nucleases (ZFNs) are now being used routinely to alter DNA sequences in many different organisms. One very crucial step in this process is the confirmation and characterization of the intended edit or change of the nucleotide sequence because the use of these genome editing mechanisms can result in the intended mutation, as well as off target mutations, or even no mutation at all. Thus, it is imperative to confirm whether the intended mutation occurred.
During genome editing, enzymes cause double-strand breaks (DSBs) at specific sites in the DNA sequence resulting in intrinsic error-prone nonhomologous endjoining (NHEJ), which leads to insertion or deletion of nucleotides (indels). NHEJ can result in imperfect repair of double-stranded breaks as observed when RNA-guided Cas9 is used (See Figure 1).

Figure 1. Development of indels during genome editing.
T7 endonuclease, an enzyme that recognizes mismatches and extrahelical loops in double-stranded DNA, is routinely used in the mismatch cleavage assay. In this assay, DNA from targeted cells potentially carrying indel-containing DNA sequences as well as intact wildtype DNA is first amplified by PCR using primers specific for the region.
During PCR, these sequences denature and reanneal forming heteroduplexes containing a mismatch (a wildtype strand and an indel-containing strand hybridize resulting in a mismatch). The amplified product is then incubated with T7 endonuclease, which recognizes the mismatches and cleaves upstream of the mismatch, while leaving the wildtype double-stranded DNA intact. The wildtype strands and resulting fragments (two or more) are then resolved in an agarose gel through electrophoresis allowing quantification of indel frequency by imaging and measuring DNA band intensity (See Figure 2)
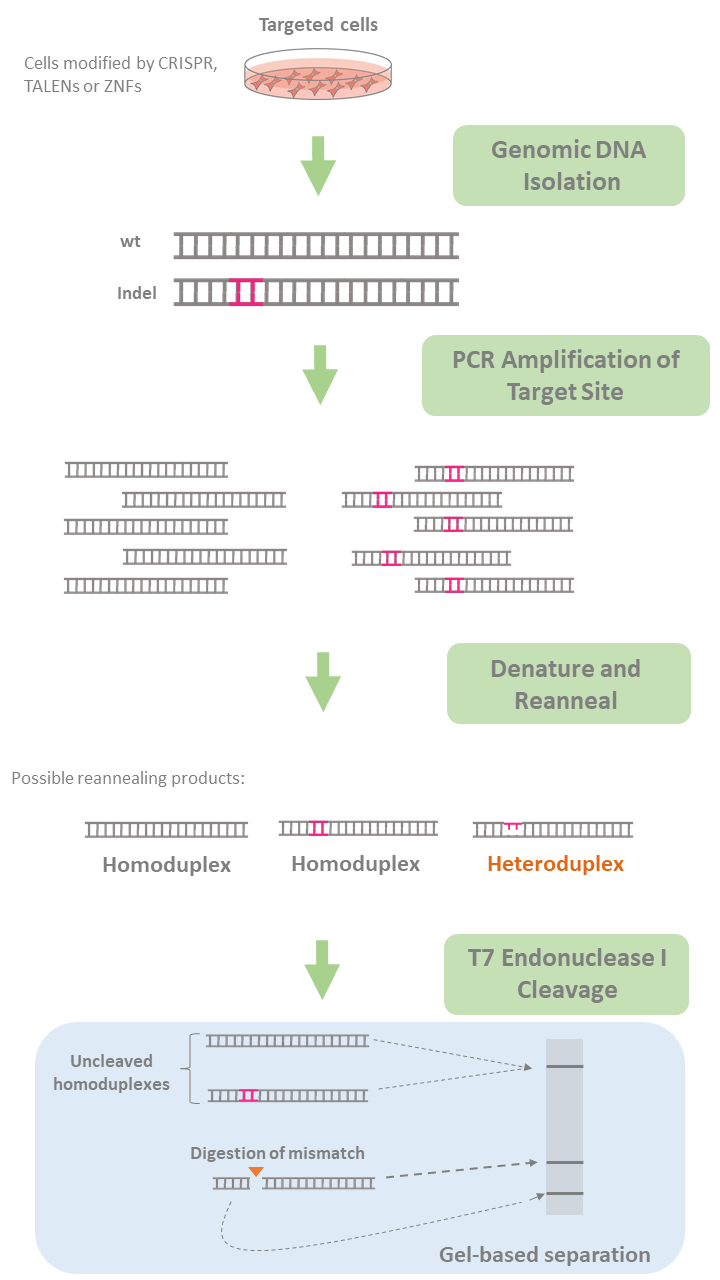
Figure 2. Evaluation of genome editing using T7 Endonuclease I.
The use of Endonuclease T7 has many advantages. It is relatively easy and inexpensive. It can be used for a few samples and can be moderately high throughput. This assay also yields results in a matter of hours and the PCR product often does not need to be purified prior to incubation with T7 endonuclease. However, this assay can only recognize small indels and cannot determine the specific mutation. In addition, T7 endonuclease can overlook changes to single nucleotides. It does not recognize single nucleotide polymorphisms (SNPs).
Moreover, its sensitivity is largely affected by reaction conditions including incubation temperature and time as well as salt concentration and amount of enzyme. In other words, optimization is often needed depending on the specific reaction conditions. Also, double-stranded DNA where both strands contain the mutation may not be easily detected in the mismatch assay causing erroneous estimation of mutation frequency.
These are general recommendations that may improve the accuracy of indel determination with T7 endonuclease.
Current DNA editing technologies are certainly facilitating the investigation of gene function. However, these technologies are only helpful if we are able to effectively determine the accuracy of mutation rate. Endonucleases such as endonuclease T7 can be powerful tools in determining targeting efficiency and off-target mutations as long as their advantages and limitations are considered.
Bloom, K., Ely, A., & Arbuthnot, P. (2016). A T7 Endonuclease I Assay to Detect Talen-Mediated Targeted Mutation of HBV cccDNA. Methods in Molecular Biology Hepatitis B Virus, 85-95. doi:10.1007/978-1-4939-6700-1_8.
Déclais, A., & Lilley, D. M. (2008). New insight into the recognition of branched DNA structure by junction-resolving enzymes. Current Opinion in Structural Biology, 18(1), 86-95. doi:10.1016/j.sbi.2007.11.001.
Guan, C. (2005). A single catalytic domain of the junction-resolving enzyme T7 endonuclease I is a non-specific nicking endonuclease. Nucleic Acids Research, 33(19), 6225-6234. doi:10.1093/nar/gki921.
Lin, Y. et al. (2014). CRISPR/Cas9 Systems Have Off-Target Activity With Insertions or Deletions Between Target DNA and Guide RNA Sequences. Nucleic Acids Research, 42(11) 7473-7485. doi:10.1093/nar/gku402.
Kim, H. J., Lee, H. J., Kim, H., Cho, S. W., & Kim, J. (2009). Targeted genome editing in human cells with zinc finger nucleases constructed via modular assembly. Genome Research, 19(7), 1279-1288. doi:10.1101/gr.089417.108.
Rivera-Torres, N., Strouse, B., Bialk, P., Niamat, R. A., & Kmiec, E. B. (2014). The Position of DNA Cleavage by TALENs and Cell Synchronization Influences the Frequency of Gene Editing Directed by Single-Stranded Oligonucleotides. PLoS ONE, 9(5). doi:10.1371/journal.pone.0096483.
Sentmanat, M. F., Peters, S. T., Florian, C. P., Connelly, J. P., & Pruett-Miller, S. M. (2018). A Survey of Validation Strategies for CRISPR-Cas9 Editing. Scientific Reports, 8(1). doi:10.1038/s41598-018-19441-8.
Vouillot, L., Thélie, A., & Pollet, N. (2015). Comparison of T7E1 and Surveyor Mismatch Cleavage Assays to Detect Mutations Triggered by Engineered Nucleases. G3|Genes|Genomes|Genetics, 5(3), 407-415. doi:10.1534/g3.114.015834.
Zischewski, J., Fischer, R., & Bortesi, L. (2017). Detection of on-target and off-target mutations generated by CRISPR/Cas9 and other sequence-specific nucleases. Biotechnology Advances, 35(1), 95-104. doi:10.1016/j.biotechadv.2016.12.003.

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...

The GoldBio Floating Tube Rack is one of our more clever giveaways because of the unique purpose it serves. And, with it also being one...

The characteristic blue color of nickel agarose beads comes from the 2+ oxidation state of the nickel ions. Color is also a useful indicator for...
