An Overview of Hydrophobic Interaction Chromatography
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
Have you ever felt as if all of your contributions in the lab go unseen and unappreciated? That if only someone would pay attention, they would see how capable and useful you are?
If so, then hydrophobic interaction chromatography (HIC) and you have a lot in common! While oft forgotten, HIC is a powerful purification technique with complementary strengths that deserves more use in our protein purification protocols.
Hydrophobic interaction chromatography (HIC) separates molecules such as proteins based on differences in their surface hydrophobicity. HIC is complementary to ion exchange chromatography in that proteins are bound to the column with high salt and eluted with low salt.
In this article we’ll discuss the principles of HIC, different types of HIC resins, biomacromolecules that can be purified in HIC, and the scenarios for which HIC is particularly useful.
How does hydrophobic interaction chromatography (HIC) work?
Hydrophobic interaction chromatography resin types
What types of biomacromolecules can you purify with hydrophobic interaction chromatography?
Positioning hydrophobic interaction chromatography in your overall purification protocol
HIC uses hydrophobic small molecules that are conjugated to agarose beads to engage hydrophobic surface patches on biomacromolecules such as proteins (Figure 1). Remember that hydrophobic literally means “water fearing.”
This term describes the “greasy” parts of molecules that would rather not mix with water and other polar and charged molecular entities.

Figure 1. Hydrophobic patches on your protein of interest (orange ovals) interact with hydrophobic chemical groups (orange circles) that are conjugated to an agarose bead.
The strength of hydrophobic interactions can be controlled by the amount of salt in your buffer. This is because hydrophobic surfaces don’t want to interact with charged salt ions, so the higher the concentration of salt the better the hydrophobic bits will bind to one another (Figure 2).

Figure 2. Salt concentration in the buffer controls the hydrophobic interaction strength for binding and release of proteins.
We leverage this relationship between salt and hydrophobic interactions in different buffers that are used to purify proteins with HIC. You’ll use relatively high salt in your binding buffer, usually 3 M sodium chloride or higher, for example. Then you’ll decrease the salt to a lower concentration, weakening the hydrophobic interactions so that your protein will elute (Figure 3).
 Figure 3. Hydrophobic interaction chromatography.
Figure 3. Hydrophobic interaction chromatography.
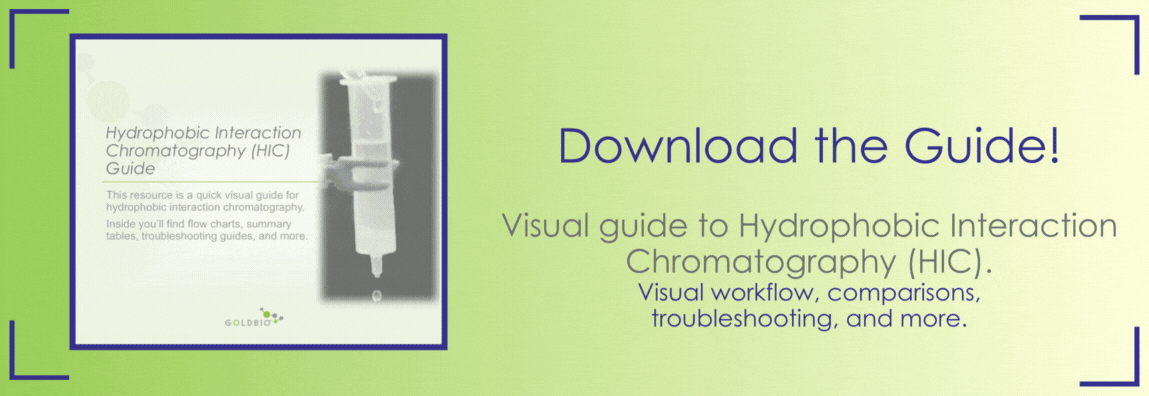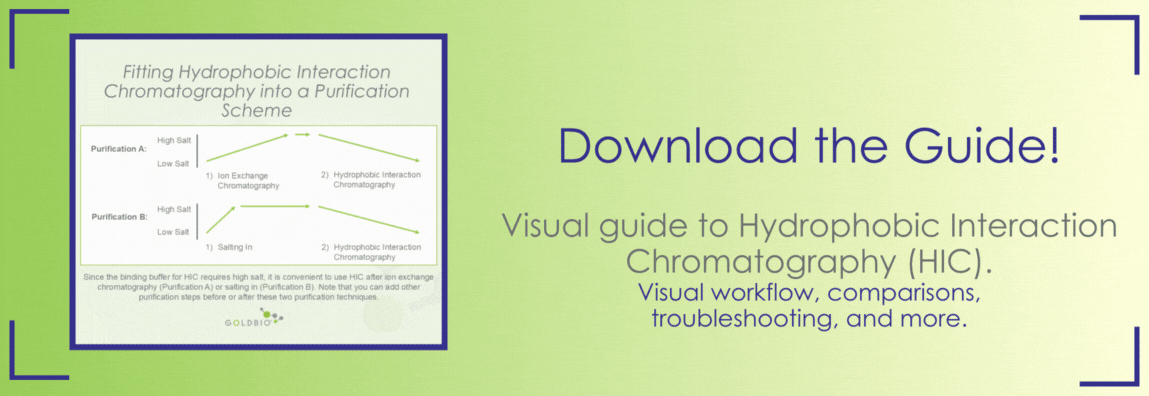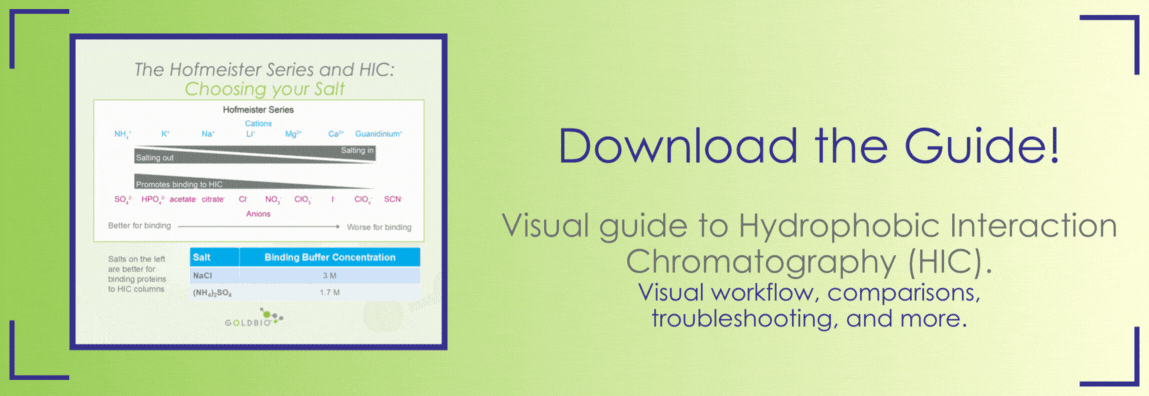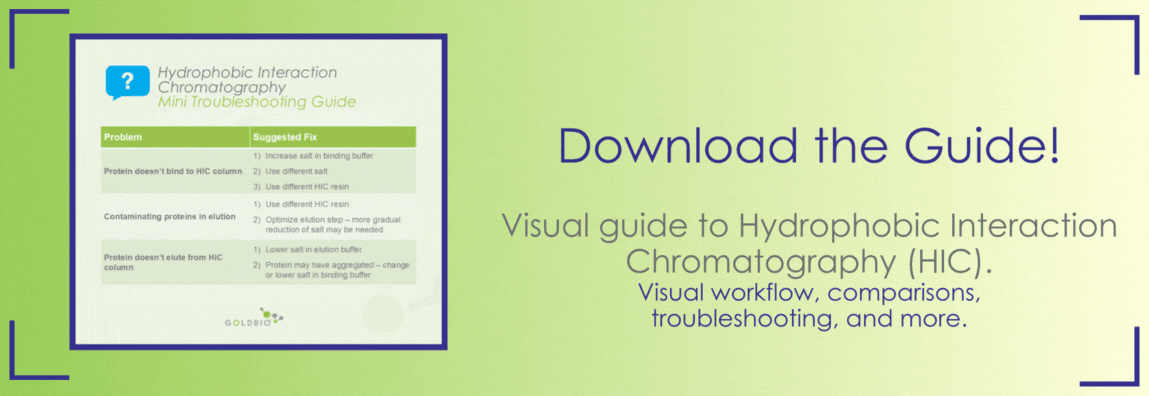
The type of salt you use will influence protein binding to the HIC resin and how much salt you need to use. The Hofmeister series describes the differential impact of distinct salts on protein stability – we discuss this series in more detail in an article about salting in and salting out proteins, so check that out if you want to learn more (Figure 4).

Figure 4. The Hofmeister series of salt ions.
With reference to HIC, ions that promote proteins salting out (towards the left of Figure 4) are more potent at driving interaction between hydrophobic proteins and HIC resin. So, the amount of salt you need to bind your protein to the HIC column will depend on the type of salt you’re using. For example, while 3 M sodium chloride is recommended for the binding buffer, only 1.7 M of the more potent ammonium sulfate is needed.
There are a number of HIC resin types that all work through the same general principle we just outlined above. The different resins most crucially differ in which hydrophobic interacting group is conjugated to the agarose beads.
For example, four commonly used chemical groups for HIC, in order of increasing hydrophobicity, are: butyl-S, octyl, butyl, and phenyl (Figure 5).

Figure 5. HIC resin types ordered by increasing hydrophobicity from left to right. Size of orange circle represents hydrophobicity, not necessarily physical size of hydrophobic chemical group.
Since phenyl is the most hydrophobic of these resins, it would typically be the best choice for proteins that are not very hydrophobic, as these proteins won’t bind very well to less hydrophobic columns like butyl-S.
Phenyl resin will also work to purify proteins with moderate to high surface hydrophobicity. These proteins will definitely bind to and elute from phenyl resin. However, for a highly hydrophobic protein you may get better resolution and purity by using a less hydrophobic resin such as butyl, octyl, or butyl-s.
In practice, many of these resins will work just fine for most proteins – so don’t stress out about which resin to use if this is your first time doing HIC with a particular protein. However, if your protein doesn’t bind to the column or if you’re dissatisfied with the purity of your eluted protein, then you can always try using a different type of HIC resin and optimizing the elution procedure.
So, for HIC the key parameters that will influence the purification are:
Usually, for a given protein the HIC resin and salt type and identity are tuned to optimize the purification. Just keep in mind that what works well for one protein, won’t always work for a different protein with distinct surface hydrophobicity (Figure 6).

Figure 6. Hydrophobic interaction chromatography can be tuned by dialing the type of HIC resin, salt concentration, and the protein of interest’s hydrophobicity.
While we’re focusing on protein purification in this article, HIC is a useful tool for purifying many biomacromolecules including:
As you can see, HIC can purify a wide range of biomacromolecules. In this article, we’re looking at HIC through the perspective of protein purification, but the same principles would apply if you were purifying an antibody or nucleic acid.
Since you’re using relatively high salt in the loading step, HIC can be a good technique to use for proteins that are unstable in low salt and are not a good fit for ion exchange. Proteins often elute at a high enough salt concentration that stability is not an issue, and if your protein is unstable in the elution buffer, you can always immediately increase the salt concentration as soon as it elutes. This limits the amount of time your protein is in low salt compared to loading your protein in a low salt buffer for ion exchange chromatography.
The binding with high salt can also be leveraged to seamlessly incorporate HIC into an overall purification scheme. For example, HIC would be a natural fit right after salting in your protein of interest, or after eluting it from an ion exchange column, since in both of those scenarios your protein is already in a high salt buffer. Consider this as a buffer-exchange step with the added benefit of the additional purity conferred by the HIC purification.
So now that we’ve gone over the basics of hydrophobic interaction chromatography, see the article links below to learn more about how HIC connects with other purification techniques and the related product links if you’re ready to start purifying proteins with HIC.

NOTE: By completing this form, you're opting in for our emails about helpful resources, GoldBio sales, and new products. And you can always opt out anytime by clicking "unsubscribe" at the bottom of a promotional message. Please review GoldBio's privacy policy.
protein purification Simon Currie

Ni2+ ions give nickel agarose beads their characteristic blue color. This blue color can fade or disappear completely when loading his-tagged proteins onto the column....

Nickel agarose beads change from blue to a brown or black color when the nickel ions have been reduced from a Ni2+ to a Ni1+...

The GoldBio Floating Tube Rack is one of our more clever giveaways because of the unique purpose it serves. And, with it also being one...

The characteristic blue color of nickel agarose beads comes from the 2+ oxidation state of the nickel ions. Color is also a useful indicator for...
