High-Throughput Methods for Protein Purification
by Simon Currie, Ph.D.

by Simon Currie, Ph.D.
Purifying proteins with traditional methods is a lot of work. To take a protein from cell lysis, through affinity and size exclusion purifications, perhaps with an ion exchange step added in the middle, is time consuming (Figure 1).
If I work hard and smart, I can purify maybe 5 to 10 proteins a week. But that’s an exhausting, labor-intensive week. Let’s consider a scenario where we wanted to purify a thousand proteins for a project. At my purification rate that would take me roughly 2-4 really exhausting years of full-time work.

Figure 1.
Standard protein purification workflow.
For campaigns designing novel proteins for a specific medical, environmental, or technical application, such as those described in the previous article, it is currently pretty common to need to test in the range of tens to thousands of proteins depending on the level of optimization needed (Cui et al, 2024; Vázquez Torres et al, 2025; Wolf et al, 2015).
That means that methods to increase throughput will be important for realizing diverse designer protein applications in an efficient manner.
High-throughput purification methods have the potential to streamline that process into a fraction of the time. There are a variety of approaches to this including:
All of these formats build on a foundation of agarose beads for protein purification. As a reminder, agarose beads are functionalized with affinity ligands or other groups to interact with proteins (Figure 2).
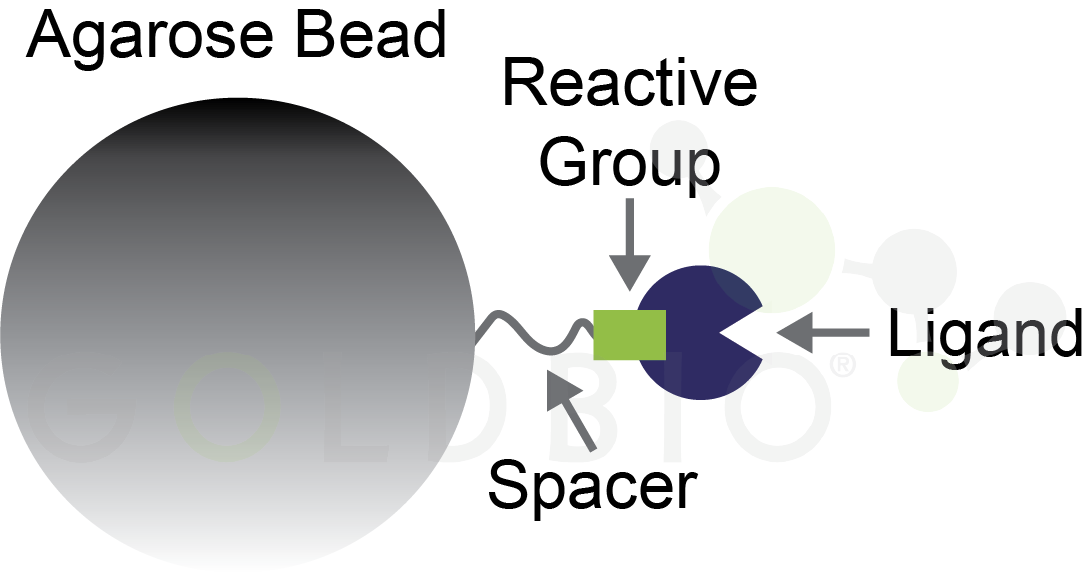
Figure 2. Agarose bead with ligand conjugated onto a reactive group for binding a specific type of protein.
Some of these applications also integrate seamlessly with E. coli protein expression to help scale overall throughput (Norton-Baker et al, 2024). Let’s dive into these methods, one-by-one, with a focus on the time it takes to purify proteins and how many proteins can be purified in parallel with these techniques.
Integrating high throughput protein purification into your research workflows
Spin columns have small resin beds and use centrifugation to force the liquid containing your protein through the resin bed which contains agarose beads (Figure 3). An advantage to this approach is that the only equipment you need is a centrifuge, something that most labs already have.
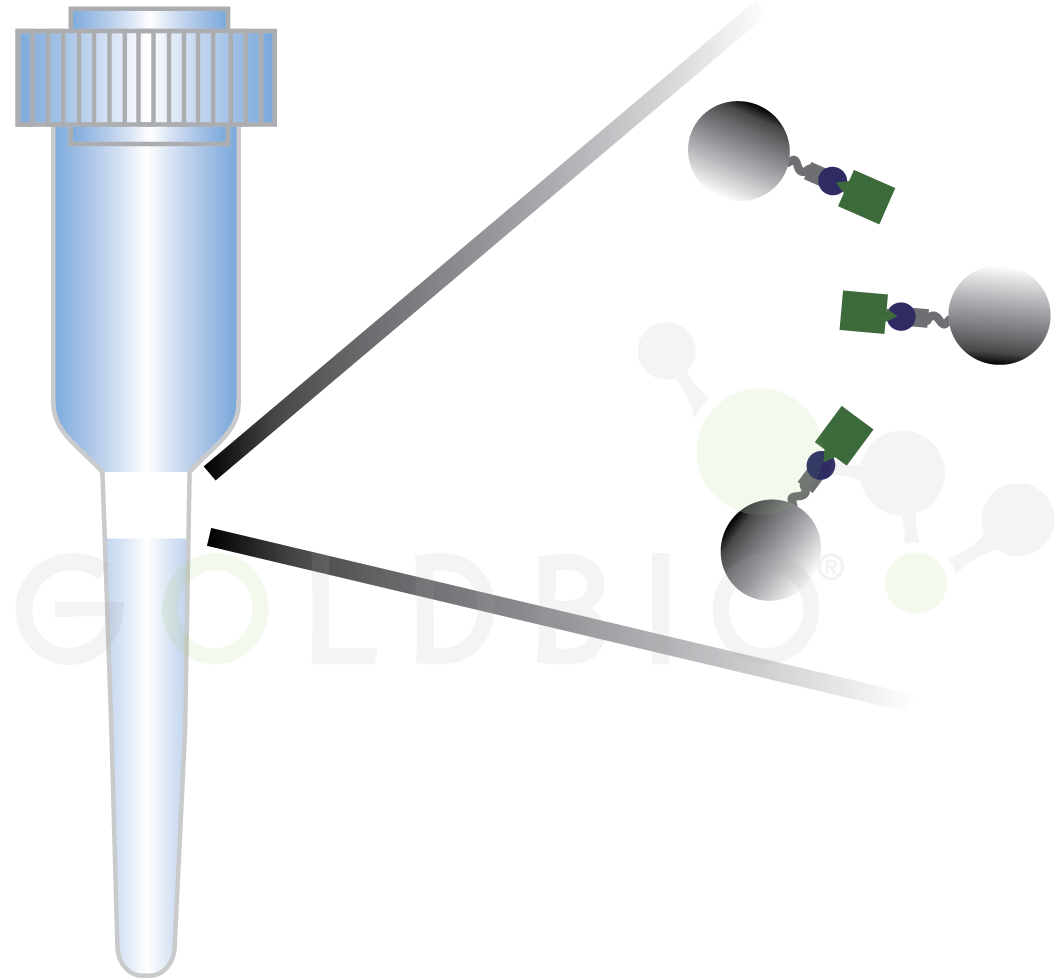
Figure 3. Spin column with agarose beads capturing protein of interest in the filter (shown magnified on the right side).
Different spin columns are available for affinity, ion exchange, hydrophobic interaction, and size exclusion purifications, so you can perform as many of these purification steps as you like with this method (Table 1).
The throughput here is essentially limited by your time and the number of slots in your centrifuge. If you have a centrifuge with 24 slots, then you could easily purify about 96 proteins per day using this method. However, compared to the tip- and plate-based formats below, this approach is time intensive and requires hands-on work by the researcher for the entire day.
Table 1. Purification types available for each method
|
Method |
Purification Types Available |
|
Spin Columns |
Affinity, Ion Exchange, Hydrophobic Interaction, Size Exclusion |
|
Magnetic Agarose Beads |
Affinity, Ion Exchange, Hydrophobic Interaction |
|
Tip-Based |
Affinity, Ion Exchange |
|
Plate-Based |
Affinity, Ion Exchange |
Tubes containing magnetic agarose beads and a protein mixture are placed into a rack with a magnet situated near the side of the tube. This magnet will attract the magnetic beads to the side of a tube, which spatially separates proteins bound to the magnetic agarose beads from the rest (Figure 4). Again, the investment cost here is pretty cheap. You just need a rack to hold your tubes with little magnetic bars on it, something that can be purchased or even custom made with a 3D printer (Figure 5) (8 Row Magnetic Rack, 2023).
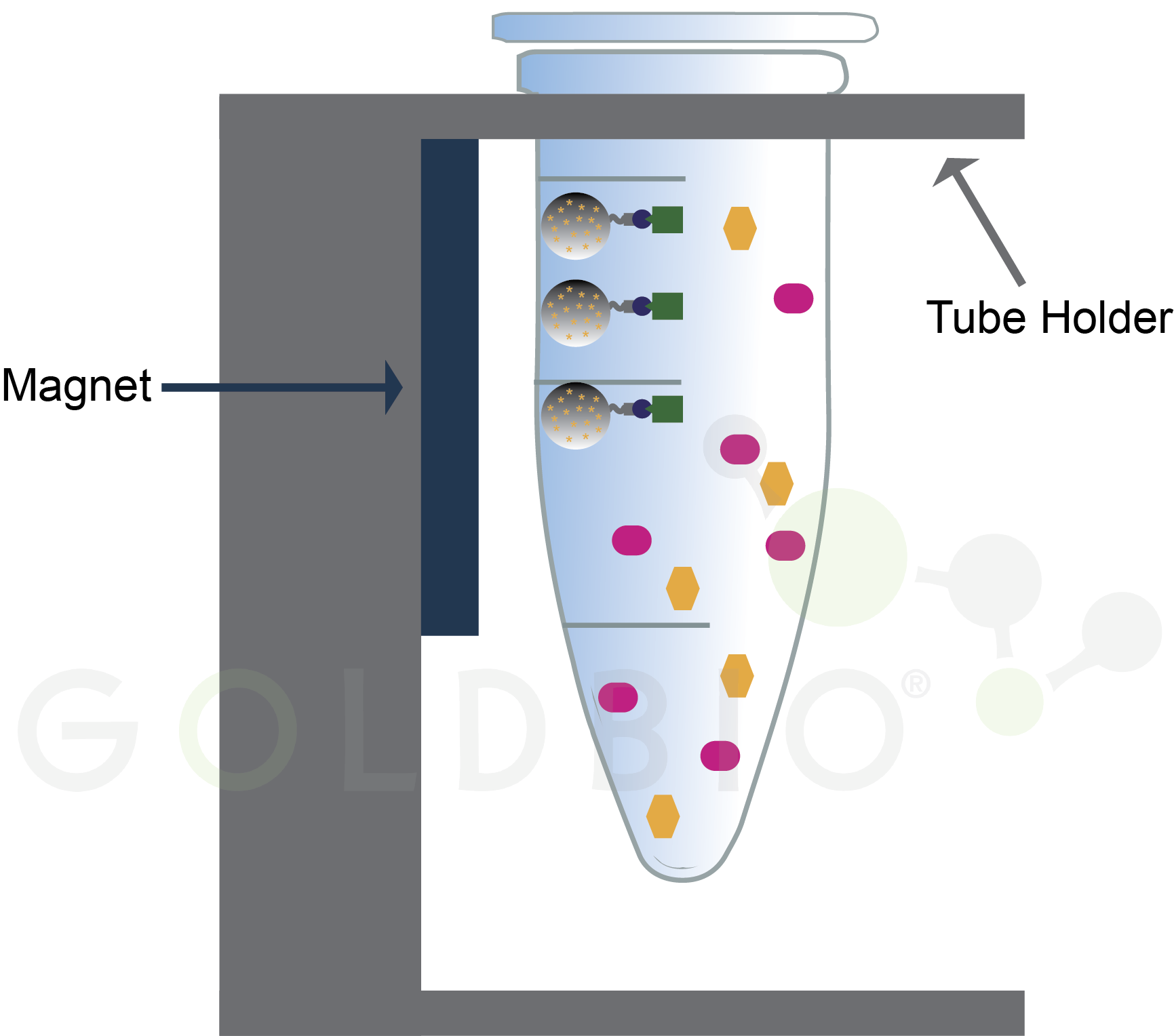
Figure 4.
Magnetic beads draw bound proteins to the side of the tube (green rectangles)
while contaminating proteins (pink ovals and orange hexagons) remain in
solution.
Magnetic beads are similar to spin columns in terms of throughput and being labor intensive. While the exact number that you get through will depend on how many slots you have in your magnetic rack, a rough estimate of ~ 100 proteins purified per day is also reasonable here, with the day being very full of hands-on work.
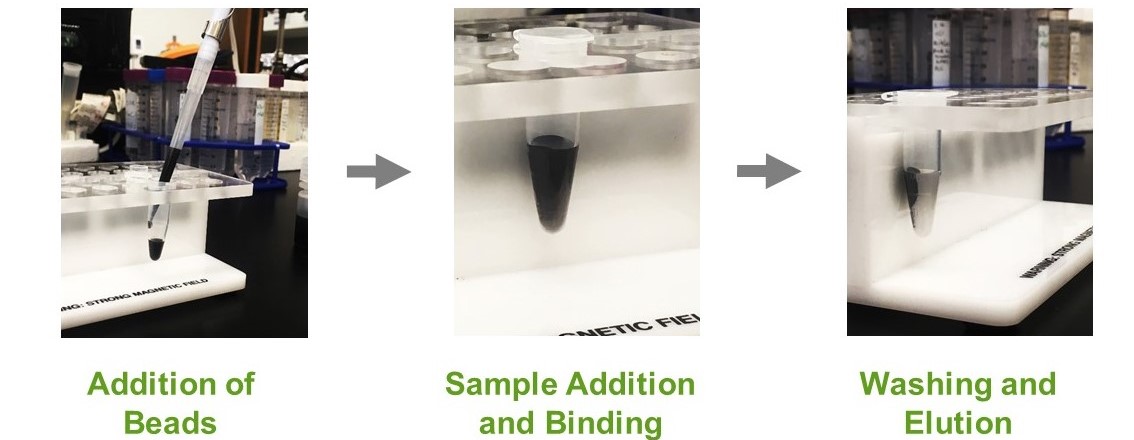
Figure 5.
Pictures of magnetic tube holder being used to purify proteins bound to
magnetic agarose beads.
Tip- and plate-based formats are useful for automating the purification using liquid handlers, which are automated instruments that pipet liquid in a high-throughput format. The benefit to using liquid handlers is that it requires much less hands-on work and increases the throughput compared to the spin columns and magnetic beads.
However, liquid handlers are a much more expensive up-front investment compared to the inexpensive equipment needed for spin columns and magnetic beads.
In tip-based formats, the protein purification occurs in resin at the bottom of a tip (Figure 6). These tips are attached to the liquid handler, and are moved into plates containing different solutions for the binding, washing, and elution steps.
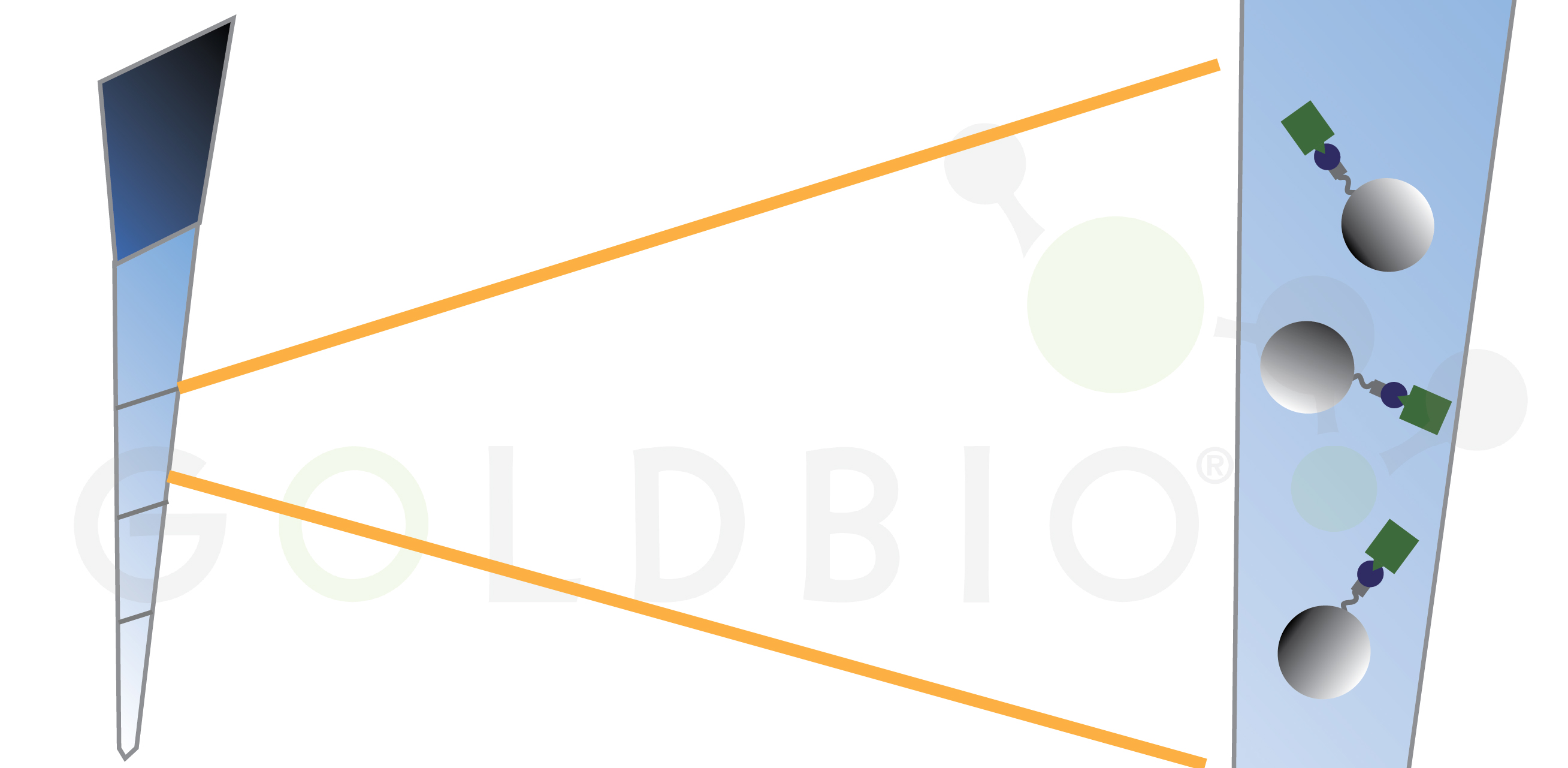
Figure 6. In
tip-based purifications, agarose beads are stored in the bottom of pipet tips
that are dipped into load, wash, and elution wells.
As I mentioned above, the higher throughput with less hands-on work is the major advantage of this format relative to spin columns and magnetic beads. One company that sells tip-based purification reagents reports that a purification takes 15 min (High-throughput Protein Purification Strategies, 2025). Assuming that you’re using a 96 well plate, then you could perform over 9,000 purifications in one day:
96 purifications per run x 4 purifications per hour x 24 hours per day = 9216 purifications per day.
96 purifications per run x 4 purifications per hour x 24 hours per day = 9216 purifications per day.
Plate-based formats are similar to tip-based formats, but in this case the resin is located in the bottom of a plate. Liquid is pipetted on top of the membrane, and then an additional “receiver” plate is placed below the filter to collect the liquid that flows through (Figure 7). This format is high-throughput, just like tip-based purifications, but again requires a liquid handler and may also need additional automation.
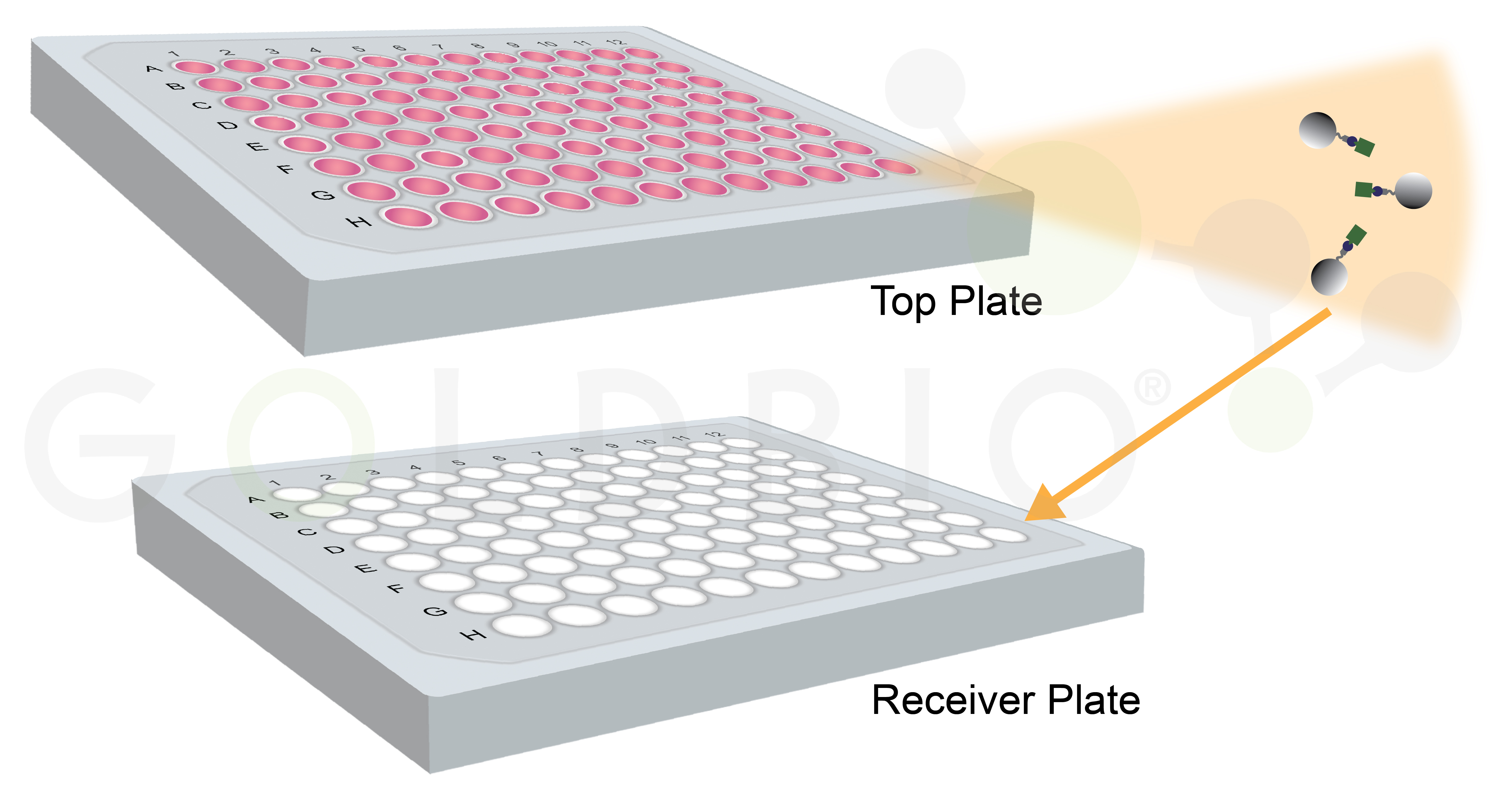
This format uses centrifugation or vacuum filtration to force the liquid from the initial plate through the membrane into the receiver plate. So, in addition to a liquid handler, you also need an automated way to centrifuge or apply a vacuum force, and to transfer the plates between these steps in order to fully realize the throughput potential of this approach.
Let’s revisit the hypothetical scenario where I need to purify 1,000 proteins. Now instead of taking a few years to purify them one by one, I can cut that time down to a few weeks with spin filters or magnetic beads, or even a single day with tip- and plate-based purification formats!
In the previous article we discussed the “design, make, test, analyze” cycle in biomedical research (Figure 8), Essentially, what we’ve done here is accelerate the make portion of this cycle, and we would now be ready to test and analyze our proteins.
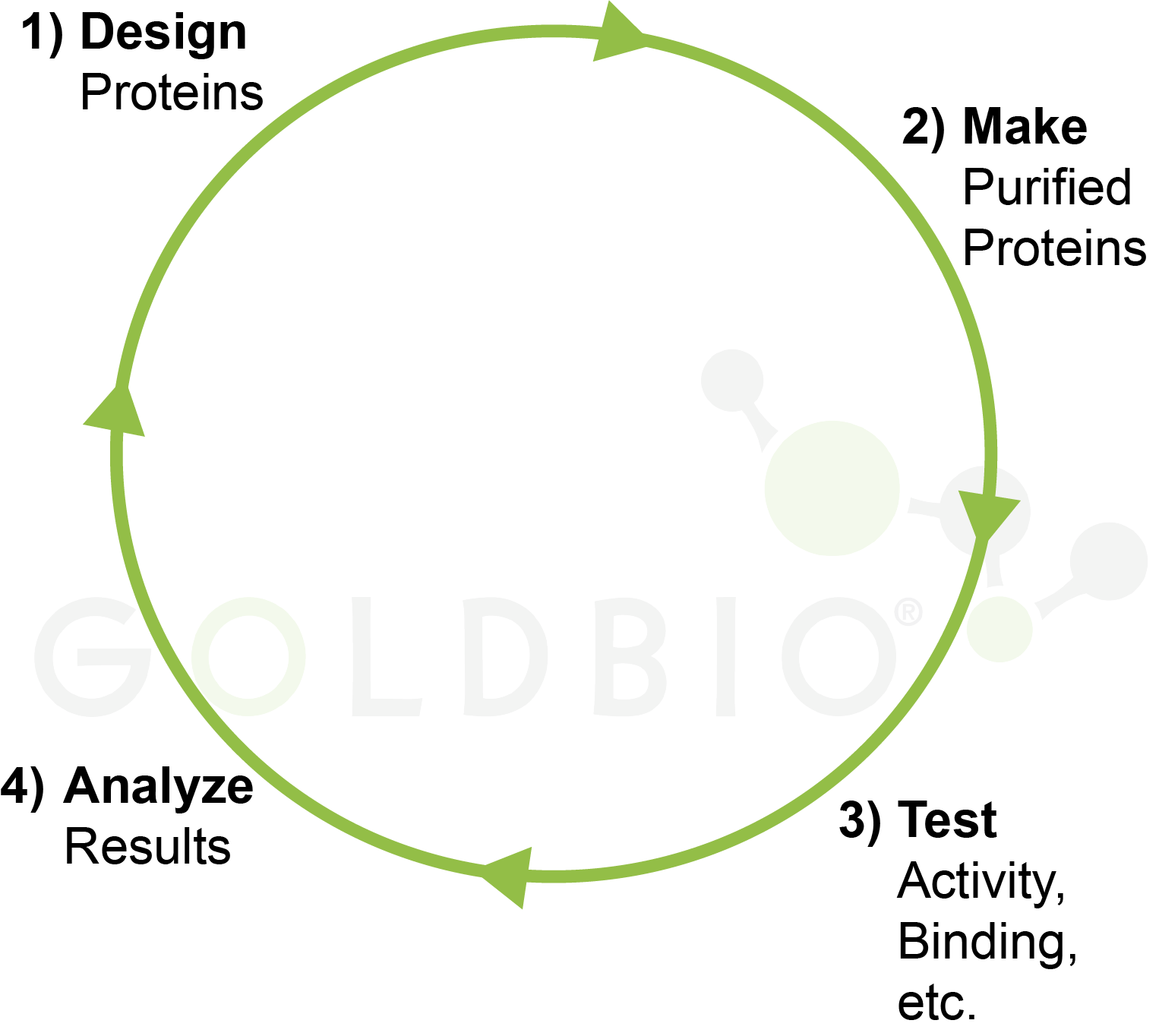
Figure 8. Design,
make, test, analyze cycle in biomedical research.
So, for example let’s say we were trying to design an enzyme with enhanced catalytic activity for a chemical reaction relative to a natural enzyme. We would now run our 1,000 designed enzymes through an assay to measure that catalytic activity and analyze that data.
In this hypothetical scenario, let’s say we had success and designed an enzyme that is 10 times more active than the original protein – hooray! However, our goal, for whatever reason, was to find an enzyme that is 100 times more active. In that case, we still have more work to do.
At this point, we would take the information from our first 1,000 designed proteins and analyze which ones worked well, and which ones performed poorly in order to design many more proteins, hoping to get to that 100-times enhanced activity threshold.
Currently, we could be using AI in the “design” stage and automation in the “make” stage to speed up this process, as we’ve discussed above. Similar automations could even assist in the “test” stage, but there is still a logjam at the analyze stage that requires lengthy human input before kicking off the whole cycle again.
All of the purification methods discussed here are currently available to enhance purification throughput in your lab. In the next article, we take a futuristic look at how these methods could be integrated into an automated and iterative “design, make, test, analyze” cycle, so check out that article if you’re interested in learning more.

The protein streptavidin clamps onto the small molecule biotin in one of the tightest natural interactions ever discovered. Over the years, the strength of this...

Maybe you are about to start working with D-luciferin, and you begin reviewing protocols. But what you’re doing is a little different. Or what you...

Handles are common in everyday life. We use them to help us open doors, cabinets, or to hold coffee mugs, cookware, and tools. They are...

Labeling antibodies with biotin or a fluorophore enables scientists to detect, track, and quantify that antibody and the molecules that it binds to. We cover...
